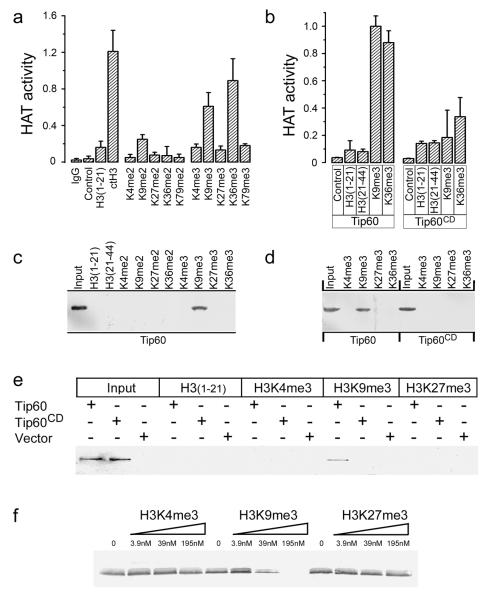
Figure 3. Tip60’s chromodomain interacts with H3K9me3 in vitro.
(a) Immunopurified HA-Tip60 was incubated with buffer (control), calf thymus H3 (ctH3), unmodified H3 peptide (amino-acids 1-21), or H3 peptide di- or trimethylated at the indicated lysine. Peptide sequences listed in supplementary table 1. Excess peptide was removed by washing, and associated Tip60 acetyltransferase activity measured. Mock immunopurification with IgG shown. Each data point represents the average of 3 independent assays, results ± SD. (b) Immunopurified HA-Tip60 or HA-Tip60CD were incubated with buffer (control), unmodified peptides H3(1-21) or H3(21-44), corresponding to amino-acids 1-21 or 21-44 of H3 respectively, or peptides trimethylated on lysine 9 (H3K9me3) or lysine 36 (H3K36me3). Each data point represents the average of 3 independent assays, results ± SD. (c) Biotinylated peptides were pre-bound to sepharose-avidin beads and incubated with nuclear extracts from cells expressing HA-Tip60. Bound Tip60 was eluted and detected by western blot using HA antibody. Unmodified peptides H3(1-21) and H3(21-44), di- or trimethylated H3K4, H3K9, H3K27, H3K36 and input Tip60 is shown. Polyacrylamide gels were stained with Coomasie Blue to confirm loading (figure S3b). (d) Biotinylated peptides were pre-bound to sepharose-avidin beads and incubated with nuclear extracts from cells expressing HA-Tip60 or HA-Tip60CD. Bound Tip60 was eluted and detected by western blot analysis using HA antibody. Polyacrylamide gels were stained with Coomasie Blue to confirm loading (figure S3c). (e) Biotinylated H3(1-21), H3K4me3, H3K9me3 or H3K27me3 peptides were pre-bound to sepharose-avidin beads and incubated with in vitro translated HA-Tip60, in vitro translated HA-Tip60CD or mock translation reactions (vector). Bound Tip60 was eluted and detected by western blot analysis using HA antibody. Input in vitro translates as indicated. (f) In vitro translated HA-Tip60 was prebound to biotinylated H3K9me3 peptide immobilized on sepharose-avidin beads. Following washing, increasing concentrations of competitor peptides H3K4me3, H3K9me3 or H3K27me3 were added and incubated for 30 minutes. The remaining HA-Tip60 was eluted from the column, separated by SDS-PAGE and detected by western blot analysis using HA antibody.