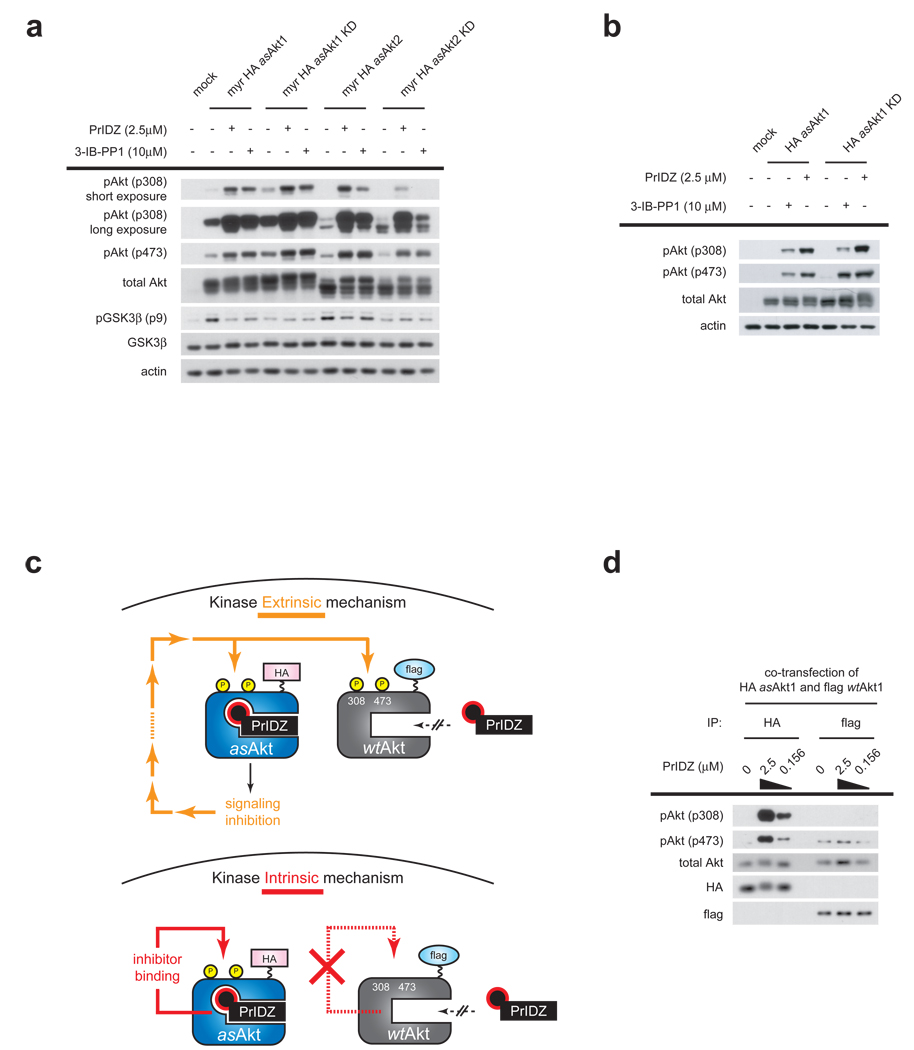
Figure 4. Hyperphosphorylation is independent of Akt signaling and results from inhibitor binding to Akt.
(a) HEK293 cells transfected with either kinase active myr-HA-asAkt1/2 or kinase dead (KD) form of myr-HA-asAkt1/2 were treated for 30 min with 2.5 µM PrINZ or 10 µM 3-IB-PP1. Cell lysates were analyzed for Akt (Thr308, Ser473) and GSK3β (Ser9) phosphorylation by immunoblotting. (b) HEK293 cells transfected with either kinase active HA-asAkt1 or kinase dead (KD) form of HA-asAkt1 were treated for 30 min with 2.5 µM PrINZ or 10 µM 3-IB-PP1. Cell lysates were analyzed for Akt (Thr308, Ser473) and GSK3β (Ser9) phosphorylation by immunoblotting. (c) Schematic representation of the expected outcomes due to extrinsic and intrinsic regulation of Akt upon treatment with PrIDZ and co-transfection of HA-asAkt1 and flag-wtAkt1. If pathway mediated feedback is causing Akt hyperphosphorylation (kinase extrinsic) then all Akt molecules in the same cell including both HA-asAkt1 and flag-wtAkt1 should be hyperphosphorylated equally. In contrast, if the occupancy of the ATP site was the only determinant of hyperphosphorylation (kinase intrinsic), then only the Akt capable of drug binding (HA-asAkt1) should be hyperphosphorylated. (d) HEK293 cells co-transfected with HA-asAkt1 and flag-wtAkt1 were treated for 30 min with various concentration of PrINZ. Cell lysates were immunoprecipitated by using either anti-HA or anti-flag antibody and then the immunoprecipitates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting.