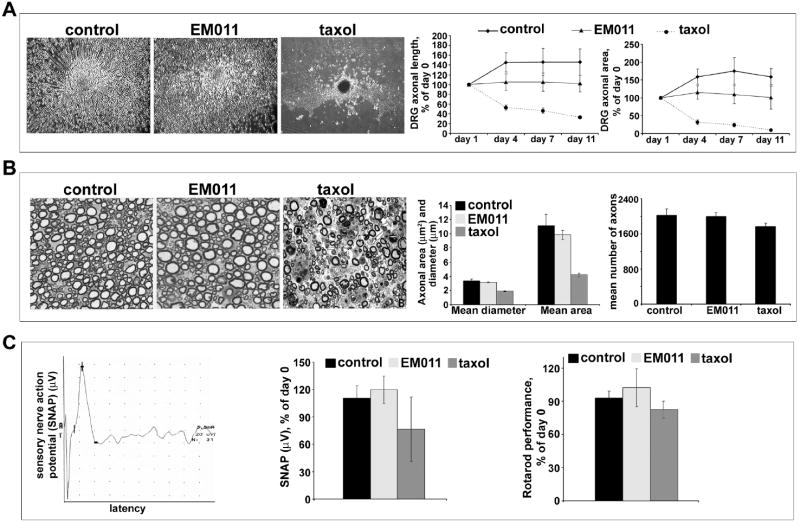
Figure 5.
Daily oral EM011 treatment does not cause any detectable peripheral neuropathy. A. Left panel shows cultured dorsal root ganglion cells treated with vehicle or EM011 or taxol. DRG cultures were allowed to mature for 5 days to enable development of a lush halo of neurites around the explants. After 5 days, cultures were incubated with vehicle or 25 μM EM011 or 30 nM taxol for up to 11 days post-treatment. The diameter of circular halo of neurites were measured on the initial day of treatment (day 0), and then on day 11. Total axonal length and the DRG halo area were the quantitative parameters used to measure neurotoxicity. Axonal survival was quantified by the longest remaining axon and the area of the remaining DRG halo. The axonal length was measured from the center of the halo to the visible distal ends of the axon in the periphery of the halo. Halo areas were calculated by tracing the outside circumference of the remaining culture halo. Percent change (of day 0) axonal length (middle) and halo area (right) upon vehicle or 25 μM EM011 or 30 nM taxol treatment for 11 days are shown. (Values are mean ± SD, P<0.05). B. Left panel shows pathological assessment of toluidine-stained 5 μm dorsal root sections imaged at 100X magnification. Middle and right panels are bar-graphical representations of the quantitation of mean diameter, area, and number of axons measured using the Image Pro software (Media Cybernetics, Silver Spring, MD). C. EM011 treatment did not cause any neurotoxicity quantifiable by electrophysiological and behavioral evaluations. Measurement of sensory nerve action potential (SNAP) in C57BL/6J mice that were treated with vehicle or 25 μM EM011 or 60 mg/kg taxol. Left panel is a representative recording of SNAP in the tail from EM011-treated mice. Middle panel shows percent change of day 0 SNAP for mice treated with vehicle or EM011 or taxol. (Values are mean ± SD, P<0.05) Right panel shows percent change in Rotarod performance (compared to day 0) of mice treated with vehicle or EM011 or taxol.