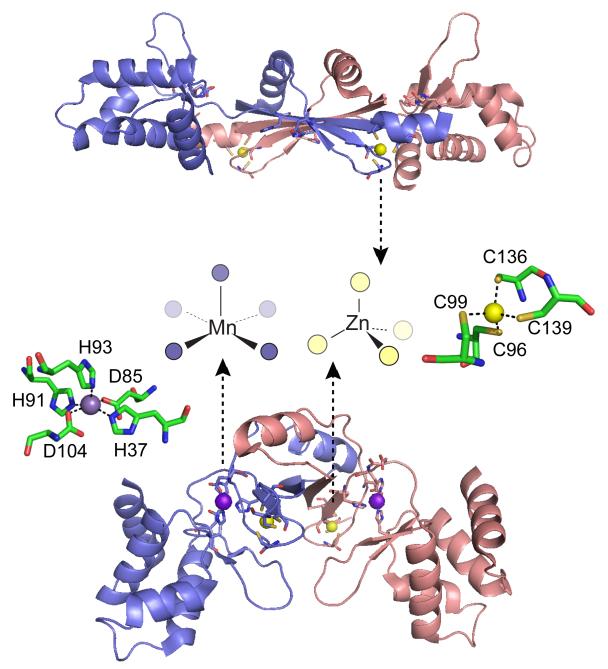
Figure 17.
Ribbon diagrams of two crystallographic structures of B. subtilis PerR with the subunits shaded blue and red.332 Top, oxidized form of PerR, designated PerR-Zn-ox, in which the regulatory metal sites are empty and H37 and H91 are modeled as 2-oxo-histidine residues.332 Each of two structural Zn(II) ions are bound to the homodimer in a tetrathiolate, tetrahedral coordination complex that is conserved in some but not all Fur family members.329 Bottom, Mn(II)-activated PerR, denoted PerR-Zn-Mn, in which the H2O2 sensing or regulatory site is formed by a square pyramidally coordinated Mn(II) or Fe(II) atom by H37 from the winged helical DNA binding domain (on the periphery of the homodimer), D85 from the connecting linker, and H91, H93 and D104 from the dimerization domain (middle), all from the same protomer. The symmetry-related metal ligands are also shown on the opposite subunit. H37 is oxidized to 2-oxo-His in PerR-Zn-oxo (shown), as is H91.326,332 The structural model of PerR-Zn-ox (top) superimposes on apo-PerR-Zn.331