Abstract
Millions of people regularly obtain insufficient sleep1. Given the impact of sleep deprivation on our lives, understanding the cellular and molecular pathways affected by sleep deprivation is clearly of social and clinical importance. One of the major effects of sleep deprivation on the brain is to produce memory deficits in learning paradigms that are dependent on the hippocampus2–5. In this study, we have identified a molecular mechanism by which brief sleep deprivation alters hippocampal function. Sleep deprivation selectively impaired cAMP/PKA-dependent forms of synaptic plasticity6 in the hippocampus, reduced cAMP signaling, and increased activity and protein levels of phosphodiesterase-4 (PDE4), an enzyme that degrades cAMP. Treatment with PDE inhibitors rescued the sleep deprivation-induced deficits in cAMP signaling, synaptic plasticity, and hippocampus-dependent memory. These findings demonstrate that brief sleep deprivation disrupts hippocampal function by interfering with cAMP signaling through increased PDE4 activity. Thus drugs that enhance cAMP signaling may provide a novel therapeutic approach to counteract the cognitive effects of sleep deprivation.
Keywords: sleep deprivation, mouse, hippocampus, memory, synaptic plasticity, cAMP, phosphodiesterase, PDE4, PDE4A5, rolipram
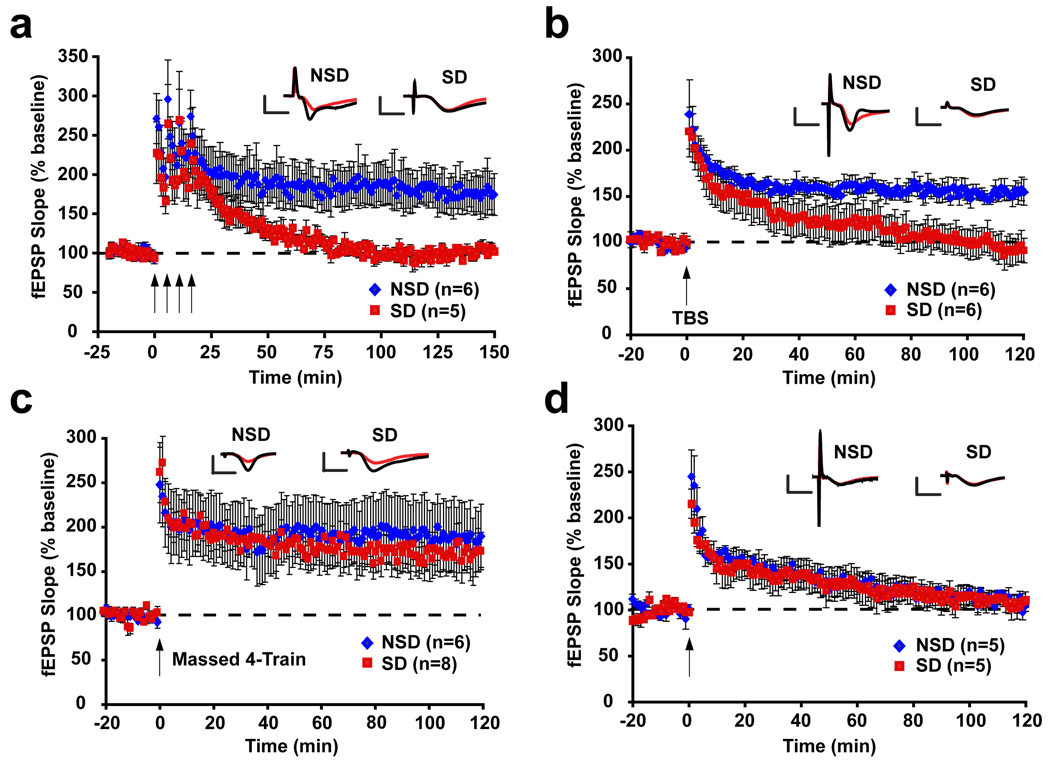
To determine the cellular and molecular mechanisms by which a short 5-hour period of sleep deprivation affects hippocampal function2, we used in vitro electrophysiological recordings to examine the effects of sleep deprivation on hippocampal long-term potentiation (LTP), a form of synaptic plasticity that relies on molecular mechanisms that are also important for memory consolidation7. We examined several forms of NMDA receptor-dependent LTP with different underlying molecular mechanisms to identify molecular targets of sleep deprivation. The long-term maintenance of LTP following spaced 4-train stimulation or theta-burst stimulation (TBS) depends on cyclic AMP (cAMP), protein kinase A (PKA), transcription, and translation8–10. Both of these forms of LTP were impaired in hippocampal slices from mice that had been deprived of sleep for 5 hours (Fig. 1a–b). The increased need for sleep that follows brief sleep deprivation dissipates in approximately 2.5 hours11, and we found that the deficit in spaced 4-train LTP also recovered with 2.5 hours of rest after 5 hours of sleep deprivation (Supplemental Fig. S4). Massed 4-train LTP, which induces a stable form of LTP that depends on translation12, but does not require cAMP/PKA signaling12,13, was unimpaired by sleep deprivation (Fig. 1c). One-train LTP, a cAMP/PKA-independent form of LTP6 was also unaffected by sleep deprivation (Fig. 1d). The lack of an effect of sleep deprivation on massed 4-train LTP and 1-train LTP suggests that brief sleep deprivation does not affect molecular mechanisms that are required for the induction and expression of these forms of LTP, such as NMDA receptor activation, Ca2+ influx and activation of Ca2+-calmodulin dependent kinase II (CaMKII)8,14,15. Because massed 4-train requires translation, the fact that sleep deprivation does not affect this form of LTP suggests that brief sleep deprivation does not generally disrupt translational processes, but instead specifically alters mechanisms that depend upon cAMP/PKA signaling. Whole-cell recordings from area CA1 confirmed that NMDA receptor function was unaffected by sleep deprivation (Supplemental Fig. S3). These results contrast with studies using longer periods of sleep deprivation, or sleep deprivation that involves exploration of a novel environment, both of which affect the initial induction of LTP as well as NMDA receptor function4,16–20. We also did not observe any effects of sleep deprivation on basal synaptic properties or short-term plasticity (Supplemental Fig. S2), suggesting that the disruption of spaced 4-train and theta-burst LTP is in fact due to disruption of signaling mechanisms underlying these forms of LTP and is not due to non-specific effects on hippocampal function.
Figure 1. Brief sleep deprivation specifically impairs forms of LTP that depend on the cAMP/PKA pathway.
(a) The maintenance of spaced 4-train LTP was significantly disrupted in slices from sleep-deprived mice (p=0.03). (b) A similar deficit was observed in LTP induced by theta-burst stimulation (TBS) (p=0.003). (c) Massed 4-train LTP was unaffected in hippocampal slices from sleep-deprived mice (p=0.67). (d) 1-train LTP was unaffected in hippocampal slices from sleep-deprived mice (p=0.97).
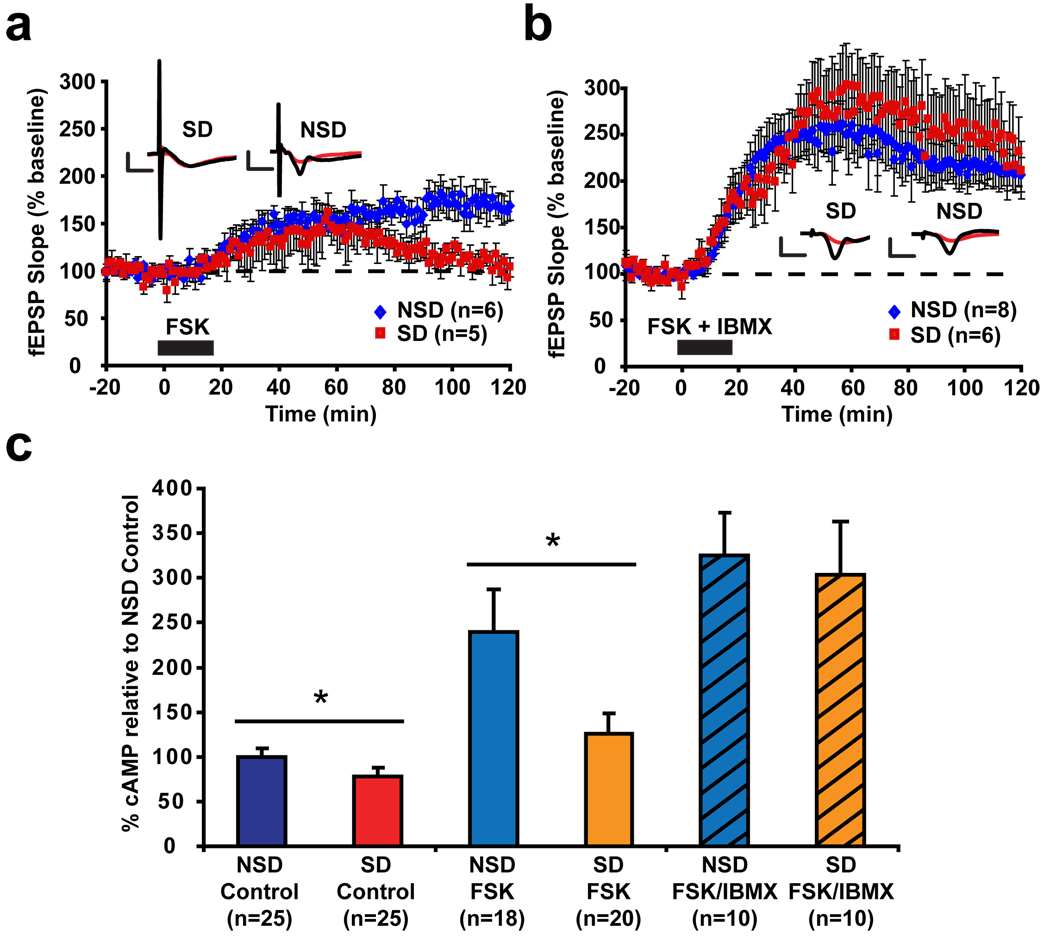
Because of the role of cAMP signaling in 4-train and theta-burst LTP8,13, we next assessed the effects of sleep deprivation on LTP induced by specific activation of the cAMP pathway using the adenylate cyclase activator forskolin. The long-term maintenance of LTP induced by treatment with forskolin (50µM) was impaired in hippocampal slices from sleep-deprived mice (Fig. 2a). Using biochemical assays, we found that baseline cAMP levels were significantly reduced in the CA1 region of hippocampal slices from sleep-deprived mice, as were cAMP levels induced by forskolin treatment (Fig. 2c). These findings demonstrate that sleep deprivation limits the ability of cells within the CA1 region of the hippocampus to respond to adenylate cyclase activation. Using immunohistochemistry, we found that phosphorylation at Ser133 of CREB, one of the downstream targets of cAMP signaling in the hippocampus21, was decreased by sleep deprivation in the CA1 and dentate gyrus (DG) regions of the hippocampus (Supplemental Fig. S7). Consistent with our published findings that brief sleep deprivation selectively impairs hippocampal function2, no effect of sleep deprivation on CREB phosphorylation was observed in the amygdala (Supplemental Fig. S7). This demonstrates that deficient cAMP signaling due to sleep deprivation impairs the activation of at least one major downstream target (CREB) in vivo. Cyclic nucleotide phosphodiesterases (PDEs) are enzymes that provide the sole route for cAMP degradation in cells22. We found that co-treatment with IBMX (30µM), a broad-spectrum inhibitor of PDEs, rescued forskolin-induced LTP as well as cAMP induction in hippocampal slices from sleep-deprived mice (Fig. 2b–c). These findings demonstrate that reversing deficits in cAMP signaling ameliorates the deficits in forskolin-induced LTP, and suggest that PDE activity might be increased by sleep deprivation.
Figure 2. Phosphodiesterase (PDE) inhibition rescues impairments in forskolin-induced cAMP levels and LTP produced by brief sleep deprivation.
(a) LTP induced by the adenylate cyclase activator forskolin (FSK) was impaired in sleep-deprived mice (SD) relative to controls (NSD) (p=0.007). (b) LTP induced by co-treatment with FSK and the PDE inhibitor IBMX was unaffected by sleep deprivation (p=0.48). (c) Sleep deprivation decreased baseline cAMP levels in CA1 regions of vehicle-treated slices (p=0.02) and significantly reduced FSK-induced cAMP levels (p=0.04). Co-application of FSK and IBMX resulted in similar cAMP levels in CA1 regions from SD and NSD mice (p=0.82).
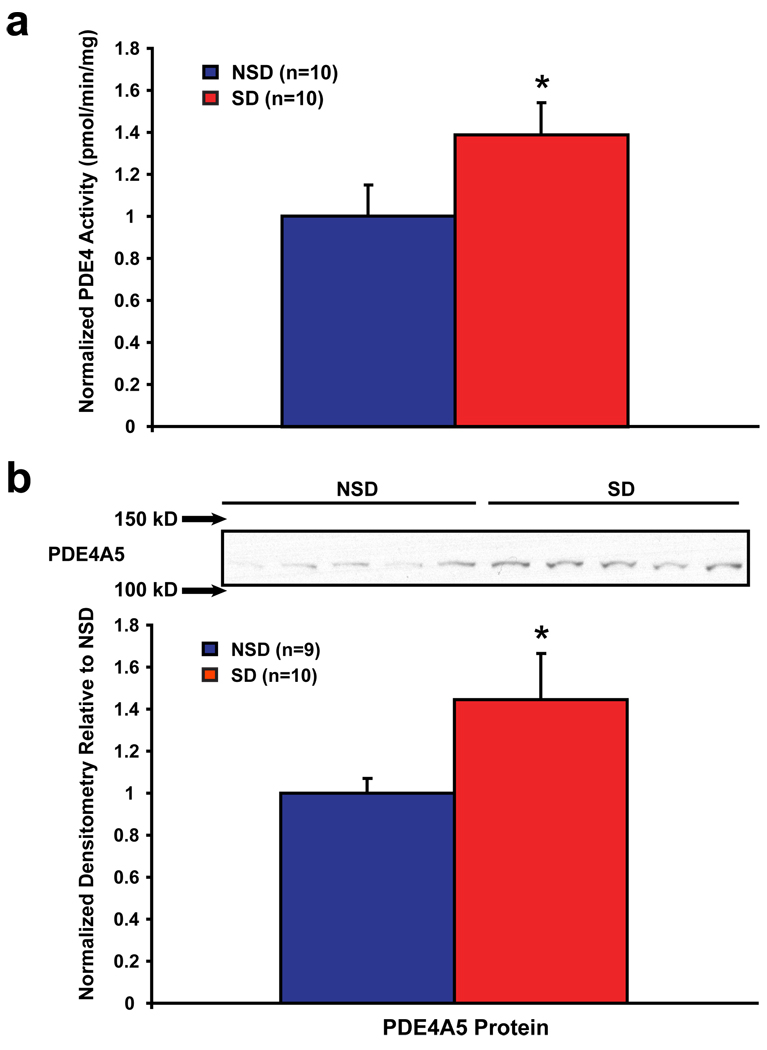
cAMP-specific PDE4 has a major role in regulating cAMP signaling in the brain22. Therefore, we tested if sleep deprivation increased PDE4 levels and/or activity in the hippocampus. We found that PDE4-specific cAMP breakdown was increased in SD mice (Fig. 3a), whereas non-PDE4 activity was unaffected (SD = 50.6 ± 2.1 pmol/mg/min, NSD = 53.4 ± 1.9 pmol/mg/min, p=0.33). In mice and humans, PDE4 isoforms are generated by four genes: PDE4A-D22. Immunoblotting with a pan-PDE4A antibody showed a trend in sleep-deprived hippocampal tissue towards an increase in a 125 kDa species, which is of a size expected for the long PDE4A5, PDE4A10 and PDE4A11 isoforms23 (Supplemental Fig. S6). Probing our hippocampal samples with antisera specific for each of these isoforms showed that PDE4A5 protein was significantly increased by sleep deprivation in the hippocampus, whereas PDE4A10 and PDE4A11 were not detected (Fig. 3b for PDE4A5; data not shown for null results with PDE4A10, PDE4A11). Interestingly, transcripts for PDE4A5 are selectively expressed in the brain with highest levels seen in hippocampal areas CA1 and DG24, the two regions in which reduction in pCREB were observed following sleep deprivation (Fig. S7). We also found that sleep deprivation elicited upregulation of PDE4 gene expression, although the pattern of changes was somewhat different between transcript and protein levels, suggesting that translation regulation mechanisms may be involved (Supplemental Fig. S5).
Figure 3. Sleep deprivation increases PDE4 activity and gene expression in the hippocampus.
(a) PDE4 activity was significantly upregulated in hippocampi from SD mice compared with NSD mice (p=0.039). (b) The PDE4 isoform PDE4A5 was significantly upregulated by sleep deprivation in the hippocampus (p=0.033). A sample blot is shown, with the nearest size markers indicated with arrows.
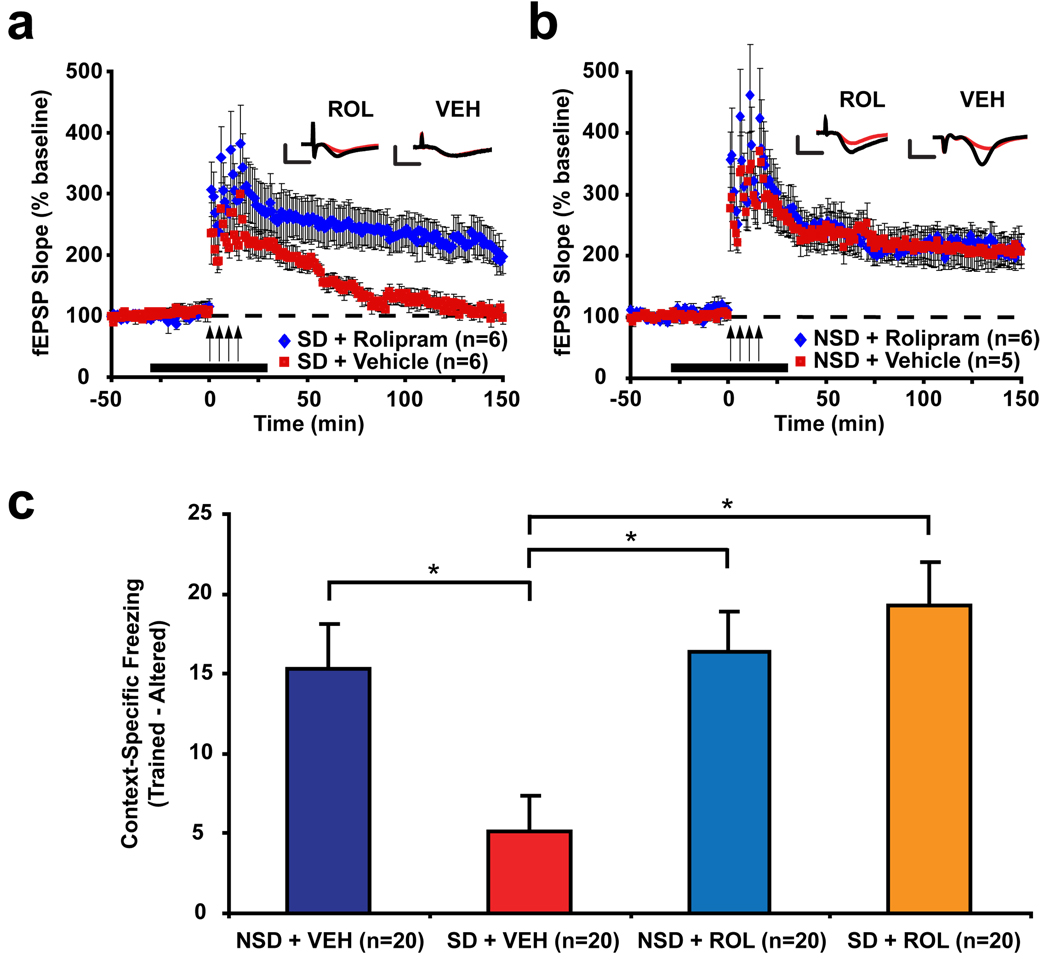
To test whether increased PDE4 activity is responsible for deficits in hippocampal function induced by sleep deprivation, we first examined whether the PDE4-selective inhibitor rolipram22 could rescue deficits in LTP induced by sleep deprivation. Indeed, rolipram treatment (0.1µM) during spaced 4-train stimulation rescued the maintenance of this form of LTP in slices from sleep-deprived mice. This dose of rolipram had no effect on 4-train LTP in slices from control mice, suggesting that this result was not simply due to a non-specific enhancement of LTP (Figs. 3a and 3b). To rule out the possibility of a ceiling effect, we assessed the effects of rolipram in slices from sleep-deprived and control mice on 1-train LTP, which is normally a transient form of plasticity. Here, rolipram enhanced the maintenance of 1-train LTP in control mice. However, rolipram had no significant effect in sleep-deprived mice, demonstrating that sleep-deprived mice have a reduced sensitivity to the effects of PDE4 inhibition (Supplemental Fig. S8). These findings suggest that sleep deprivation makes it more difficult to reach the threshold for induction of cAMP/PKA-dependent LTP, consistent with our observation that sleep deprivation increases the activity of a specific PDE4 isoform that is known to set the threshold for activation when recruited to specific signaling complexes25.
We next assessed whether in vivo PDE4 inhibition could prevent impairments in hippocampus-dependent memory consolidation induced by post-training sleep deprivation. Immediately following single-trial contextual fear conditioning, mice were either deprived of sleep by gentle handling for 5 hours or were left undisturbed in their home cages. Mice received 2 IP injections of either rolipram (ROL; 1 mg/kg) or vehicle (VEH), immediately and 2.5 hours following training. The timing of these injections was based on data showing that the effects of rolipram on hippocampal cAMP levels last for about 3 hours (personal communication, Dr. James O’Donnell). Memory was assessed using a 5 minute retrieval test in the trained context 1 day after conditioning and in an altered context 2 days after conditioning. Context-specific memory, a measure that is particularly dependent on hippocampal function26, was measured by subtracting the percent freezing in the altered context from the percent freezing in the trained context (see Supplemental Fig. S9 for data from these two tests). Sleep deprivation significantly impaired context-specific memory, and rolipram treatment rescued this deficit (Fig. 4c). The ability of rolipram to rescue deficits in hippocampus-dependent memory produced by sleep deprivation demonstrates that disruption of cAMP signaling is responsible for the effects of sleep deprivation in vivo.
Figure 4. The PDE4 inhibitor rolipram rescues LTP and memory deficits caused by sleep deprivation.
(a) Rolipram (ROL) treatment rescued deficits in spaced 4-train LTP due to sleep deprivation (p=0.003). (b) However, rolipram showed no further enhancement of spaced 4-train LTP in NSD mice (p=1.0). The black bar in (a) and (b) represents the time of ROL treatment. (c) Sleep deprivation significantly impaired context-specific memory (p=0.02), and treatment with rolipram rescued this deficit (p=0.0009) without affecting memory in non-sleep-deprived mice (p=0.99).
A major challenge in the field of sleep research has been to determine how the sleep disruptions associated with neurological and psychiatric disorders, aging, and everyday living affect cognitive function. These findings are the first to define a molecular mechanism underlying the effects of sleep deprivation on hippocampal function at the behavioral and synaptic level, and they lay the groundwork for further analysis of the functional biochemistry of sleep deprivation and the development of novel therapeutics to ameliorate the impact of sleep deprivation. These experiments using the PDE4 inhibitor rolipram are the first to rescue synaptic plasticity and memory deficits produced by a brief period of sleep deprivation. Our data suggest that if compounds can be developed that block either the activity, expression, or targeting of the PDE4A5 isoform, they may prove useful in the treatment of the cognitive effects of sleep deprivation. Interestingly, circadian oscillation of cAMP in the hippocampus has recently been linked to the persistence of memory27 so such drugs may also be useful in treating memory deficits associated with alterations in circadian rhythms. It has proven difficult to generate active-site directed inhibitors showing selectivity for each of the four PDE4 sub-families because of the highly conserved structure of their catalytic unit25, but it may be possible to develop compounds that disrupt the targeting of specific PDE4 isoforms from appropriate scaffolds in cells22,25.
Methods Summary
C57BL/6J male mice (2–5 months of age) were housed individually on a 12 hr/12 hr light/dark schedule with lights on at 7 A.M. (ZT0) and handled for 6 days. Mice were sleep-deprived (SD) in their home cages for 5 hours by gentle handling beginning at ZT5 or left undisturbed (non-sleep-deprived mice, NSD). For contextual fear conditioning experiments, animals were placed in a novel chamber for 3 minutes, and received a 2-second, 1.5 mA footshock after 2.5 minutes. Half of the mice were deprived of sleep for 5 hours post-training. Mice received intra-peritoneal injections of rolipram (ROL; 1 mg/kg) or vehicle (2% DMSO in 0.9% saline) immediately and 2.5 hours post-training. Testing of contextual memory was performed 24 hours after training in the trained context and 48 hours after training in a novel chamber.
Electrophysiological recordings were carried out as previously reported28. 1-train LTP was induced by a single 100 Hz, 1-second duration train of stimuli. 4-train LTP consisted of 4 trains applied with a 5-minute inter-train interval; for massed 4-train LTP a 5-second inter-train interval was used. Theta-burst stimulation (TBS) consisted of 40-ms duration, 100 Hz bursts delivered at 5 Hz for 3 seconds (15 bursts of 4 pulses per burst, for a total of 60 pulses). Chemical LTP was induced by treatment of slices for 15 minutes with 5µM forskolin (FSK) in 0.1% ethanol, or a combination of 50µM forskolin and 30µM 3-isobutyl-1-methylxanthine (IBMX, in water). Rolipram (0.1µM in 0.1% DMSO) was applied for 60 minutes, beginning 30 minutes before tetanization.
cAMP assays on CA1 regions of hippocampal slices 10 minutes after treatment for 15 minutes with forskolin (50µM), forskolin + IBMX (30µM), or vehicle (0.1% EtOH) were performed by radioimmunoassay according to kit instructions. cAMP-specific PDE activity assays29 and Western blots for PDE4A530 were performed as previously described.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Material
Acknowledgements
We thank Dr. Amita Sehgal and Joshua Hawk for their comments on the manuscript. We thank Dr. James O’Donnell for his help with planning rolipram treatment experiments. We thank Dr. James Bibb for helpful discussions and comments on the manuscript. We thank Dr. Steven Fluharty and Dr. Jon Lindstrom for the use of their gamma scintillation counters, and Dr. Tracy Bale, Dr. Nirupa Goel, Dr. Kate Semsar, and Dr. Sarah Teegarden for their help with corticosterone assays. We also thank Dr. Pepe Hernandez, Nora Khatib, Alan Park, James Lederman, Dr. Cedrick Florian, and Dr. Wei Lu for their help with experiments. This research was supported by Systems and Integrative Biology Training Grant GM07517 (To C.G.V.; M. Nusbaum P.I.), NIH training grant HL07953 (to C.G.V.; A.I. Pack P.I.), the Netherlands Organization for Scientific Research NWO-Rubicon Grant 825.07.029 (to R.H.), the National Institutes of Health (AG017628) (to T.A.; A.I. Pack, P.I.), SCOR Grant HL060287 (to T.A., A.I. Pack, P.I.), HFSP grant RGSP/2005 (to T.A.), the Medical Research Council (UK) grant G0600765 (to M.D.H. and G.S.B.), the European Union grant LSHB-CT-2006-037189 (to M.D.H.), the Fondation Leducq grant 06CVD02 (to M.D.H. and G.S.B.), CIHR84256 (to M.Z.), and a UK Engineering and Physical Sciences Research Council training grant (to K.M.B.).
Footnotes
Author Contributions
Experiments were conceived and designed by C.G.V., T.A., G.S.B., M.D.H., and M.Z. Behavioral and gene expression experiments were carried out by D.J. and C.G.V. Electrophysiological recordings were carried out by C.G.V. and T.H. cAMP assays were carried out by C.G.V. Western blots were carried out by K.M.B. PDE activity assays were carried out by G.S.B. Immunohistochemistry experiments were carried out by R.H. and A.D. EEG/EMG recordings were carried out by M.W. Sleep deprivation and electrophysiology for whole-cell patch clamp recordings were performed by X.-Y.L., G.D., S.S.K., T.C., and Y-Z.S. Manuscript was prepared by C.G.V. and T.A., with input from D.J., R.H., M.W., G.S.B. and M.D.H.
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
References
- 1.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Insufficient sleep--a population-based study in adults. Sleep. 2001;24:392–400. doi: 10.1093/sleep/24.4.392. [DOI] [PubMed] [Google Scholar]
- 2.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain research. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 4.McDermott CM, et al. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 8.Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 9.Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science (New York, N.Y. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen PV, Kandel ER. Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem. 1997;4:230–243. doi: 10.1101/lm.4.2.230. [DOI] [PubMed] [Google Scholar]
- 11.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain research. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 12.Scharf MT, et al. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- 13.Woo NH, Duffy SN, Abel T, Nguyen PV. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- 14.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 15.Lynch G, Kessler M, Halpain S, Baudry M. Biochemical effects of high-frequency synaptic activity studied with in vitro slices. Fed Proc. 1983;42:2886–2890. [PubMed] [Google Scholar]
- 16.Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. The Journal of physiology. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tartar JL, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. The European journal of neuroscience. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- 22.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace DA, et al. Identification and characterization of PDE4A11, a novel, widely expressed long isoform encoded by the human PDE4A cAMP phosphodiesterase gene. Mol Pharmacol. 2005;67:1920–1934. doi: 10.1124/mol.104.009423. [DOI] [PubMed] [Google Scholar]
- 24.McPhee I, Cochran S, Houslay MD. The novel long PDE4A10 cyclic AMP phosphodiesterase shows a pattern of expression within brain that is distinct from the long PDE4A5 and short PDE4A1 isoforms. Cellular signalling. 2001;13:911–918. doi: 10.1016/s0898-6568(01)00217-0. [DOI] [PubMed] [Google Scholar]
- 25.Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 26.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 27.Eckel-Mahan KL, et al. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nature neuroscience. 2008 doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobban M, Shakur Y, Beattie J, Houslay MD. Identification of two splice variant forms of type-IVB cyclic AMP phosphodiesterase, DPD (rPDE-IVB1) and PDE-4 (rPDE-IVB2) in brain: selective localization in membrane and cytosolic compartments and differential expression in various brain regions. Biochem J. 1994;304(Pt 2):399–406. doi: 10.1042/bj3040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huston E, et al. The cAMP-specific phosphodiesterase PDE4A5 is cleaved downstream of its SH3 interaction domain by caspase-3. Consequences for altered intracellular distribution. J Biol Chem. 2000;275:28063–28074. doi: 10.1074/jbc.M906144199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.