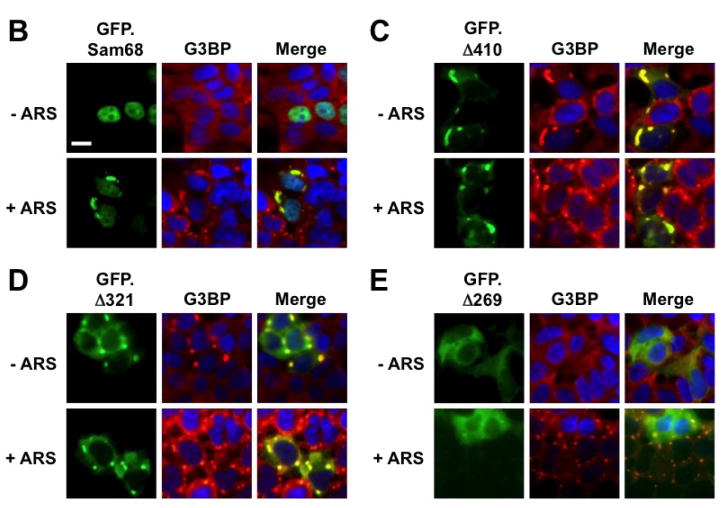
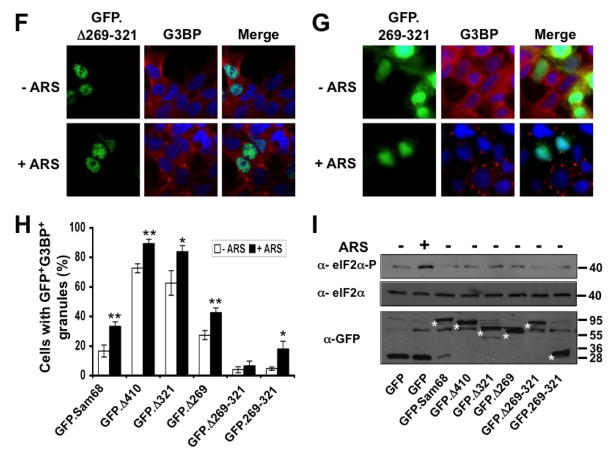
Figure 3. Requirement of specific Sam68 domain for its SG recruitment.
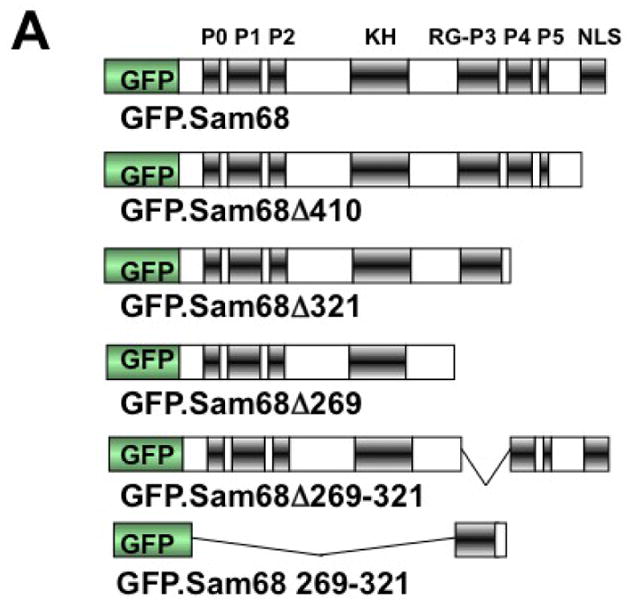
A. P: proline-rich domain; KH: KH domain; RG: arginine-glycine rich domain; and NLS: nuclear localization signal. The single lines in GFP. 269–321 and GFP.269–321 represent deleted regions. B–G. 293T cells were transfected with GFP-tagged Sam68 (B), deletion mutants containing various lengths of C-terminal deletion, i.e. Δ410 (C), Δ321 (D) or Δ 269 (E), Δ269–321 lacking the domain of aa269–321 (F), or 269–321 expressing the domain of aa269–321 (G). Cells were cultured for 48 hr and then treated with 0.5 mM ARS (+ ARS) or without ARS (− ARS) for 1 hr prior to fixation. The cells were then stained using a mouse α-G3BP antibody followed by PE-conjugated goat α-mouse secondary antibody. The images were representative of each transfection. Co-localization of G3BP with Sam68 or each mutant was shown in the column marked as “Merge”. H. Quantitation of GFP+G3BP+ cells in each transfection in B–G, which was expressed as a percentage of the total number of GFP+ cells. The data were mean ± SEM of at least three independent experiments. The comparison was made between ARS-treated and untreated cells. *: p < 0.05; **: p < 0.05. I. 293T cells were transfected with GFP-tagged Sam68 or each of its mutants as indicated, followed by Western blot analysis using α-eIF2α, α-phosphorylated eIF2α (α-eIF2α-P), or α-GFP antibodies. Cells that were transfected with GFP and treated with 0.5 mM ARS or without ARS were included as positive and negative controls for eIF2α phosphorylation, respectively, in the Western blot analysis. *: GFP-tagged proteins.