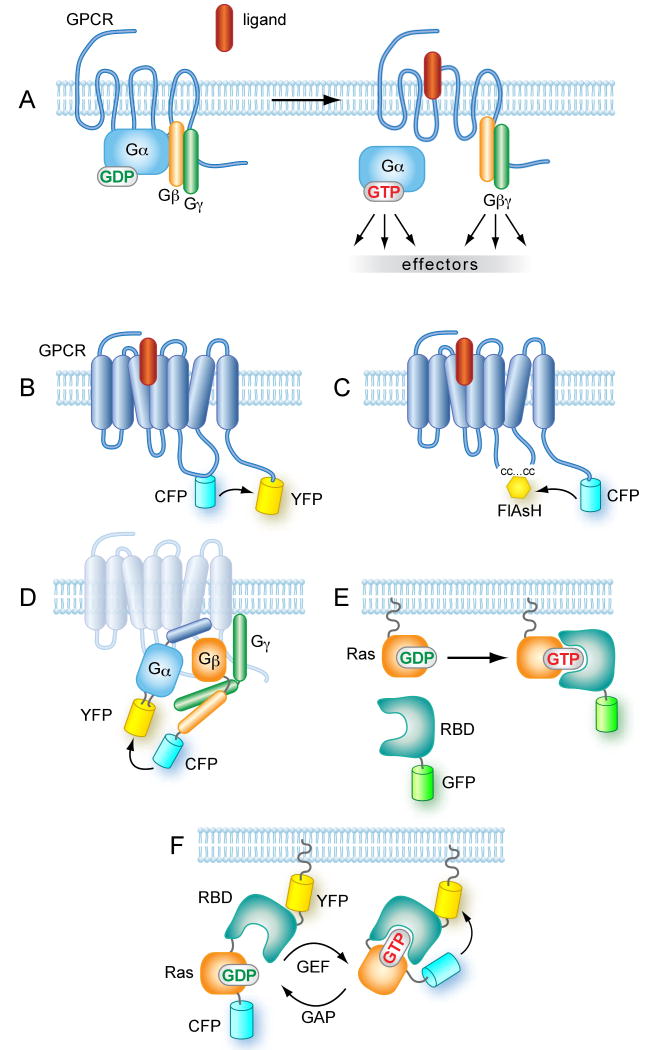
Figure 2. Schematics of the designs of fluorescent probes to follow GPCR and G-protein activation.
(A) Early signaling from GPCRs. In quiescent cells the receptor is associated with the heterotrimeric G protein complex composed of an α-subunit and a tightly associated βγ heterodimer. Ligand binding induces a conformational change in the receptor leading to the dissociation of Gα- and Gβγ subunits. The GDP/GTP exchange on the α-subunit results in a Gα that can activate a number of downstream effectors, such as adenylate cyclases, but the freed Gβγ dimmer is also capable of stimulating effectors molecules (B) GPCRs tagged at the C-terminus and within the 3rd intracellular loop with properly matched fluorescent proteins can detect the ligand-induced conformational change in the form of change in FRET efficiency. However, such designs can severely affect coupling of the tagged receptors to G proteins. Black arrow indicates direction of energy transfer. (C) Using smaller fluorescent tags in the 3rd loop such as the tetracysteine (‘CC…CC’) tag reacted with FlAsH, can alleviate this problem. Black arrow indicates direction of energy transfer. (D) Dissociation and/or conformational change of heterotrimeric G proteins can be monitored by FRET. Here one fluorophore is placed on the N- or C-terminus of either the Gβ or the Gγ subunits. The placement of the fluorescent molecule within the Gα subunit is a more delicate task, but successful FRET was achieved when GFP was placed between the A and B α-helices. It is important that the fluorescent protein does not interfere with the lipid modification and hence, membrane attachment of the heterotrimeric complex 116. Black arrow indicates direction of energy transfer. (E) Active (GTP-bound) forms of small GTP binding proteins can be detected by recruitment of fluorescent fusion proteins containing a recognition domain such as the RBD of Raf-1. This approach poorly detects endogenous Ras activation but has been used to monitor the activation of overexpressed Ras proteins. (F). The FRET probes to monitor activation of small GTP binding proteins are based on the design that incorporates both the small G protein and a domain recognizing its GTP-bound form sandwiched in between the two fluorescent proteins. This probe is then targeted to a membrane of interest where it registers the change in the sum of GEF and GAP activities. See text for original citations.