Table 1.
Asymmetric Epoxidation of Non-conjugated cis-Olefinsa
| entry | substrate | product | ketone | temp (°C) | time (h) | yield (%)b | ee (%) |
|---|---|---|---|---|---|---|---|
| 1c |  |
2d | 0 | 8 | 52 | 64d,8 | |
| 2 |  |
 |
2d | −10 | 8 | 71 | 79e,f,12 |
| 3 | 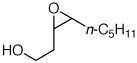 |
2d | −10 | 4 | 89 | 82e,13 | |
| 4 |  |
2d | −10 | 8 | 75 | 91d,f,11h | |
| 5 |  |
 |
2d | −10 | 8 | 85 | 86g,14 |
| 6 |  |
 |
2a | −10 | 8 | 79 | 61d,h |
| 7i,j,k |  |
 |
2a | −10 | 12 | 73 | 86d |
| 8i,j,k |  |
 |
2a | −10 | 12 | 71 | 85d,h |
| 9 |  |
 |
2a | −10 | 12 | 80 | 32l |
| 10i,j |  |
2a | −10 | 12 | 76 | 92d | |
| 11i,j |  |
 |
2a | 0 | 12 | 39 | 51d |
| 12 |  |
 |
2a | 0 | 8 | 87 | 59d,5b,15 |
For ketone 2d unless otherwise stated, all reactions were carried out with olefin (1.0 equiv), catalyst (0.25 equiv), Oxone (1.6 equiv), and K2CO3 (6.7 equiv) in DME/DMM (3:1, v/v) and buffer (0.1 M K2CO3-AcOH in 4 × 10−4 M aq EDTA, pH 9.3) (1.5:1, v/v). For ketone 2a unless otherwise stated, all reactions were carried out with olefin (1.0 equiv), catalyst (0.25 equiv), Oxone (1.6 equiv), and K2CO3 (3.8 equiv) in DME/DMM/n-BuOH (3:1:2, v/v/v) and buffer (0.1 M K2CO3-AcOH in 4 × 10−4 M aq EDTA, pH 8.0) (4:1, v/v). In both cases Oxone and K2CO3 were added separately and simultaneously over the time and temperature specified.
Isolated yield.
DME was used as solvent.
The ee was determined by chiral GC (Chiraldex B-DM column).
The ee was determined by chiral GC (Chiraldex B-DM column) of the methyl ether derivative.
Absolute stereochemistry was determined by comparing the optical rotation of the epoxide or its derivate with the reported one.
Relative stereochemistry indicated. The ee was determined by chiral HPLC (Chiralpak AD column) of the benzoate derivative.
Absolute stereochemistry was determined by converting a compound of known configuration to the epoxide of interest and comparing the optical rotation and chiral GC elution order.
0.30 equiv catalyst was used.
2.9 equiv Oxone and 6.9 equiv K2CO3 were used.
DME/DMM (3:1, v/v) was used as solvent; the solvent/buffer ratio was 1.5:1 (v/v).
The ee was determined by chiral HPLC (Chiralcel OD column).
