Abstract
Background
Symptoms of depression and anxiety overlap strongly and are independent predictors of cardiovascular disease (CVD) events. Although these symptoms commonly co-occur in medical patients, little is known about combined effects of depression and anxiety on CVD risk.
Objective
To study the independent and interactive effects of depression and anxiety symptoms as predictors of CVD events in a sample of women with suspected myocardial ischemia.
Method
A total of 489 women completed a baseline protocol including coronary angiogram, CVD risk factor assessment, and questionnaire-based measures of depression and anxiety symptoms using the Beck Depression Inventory (BDI) and State Trait Anxiety Inventory (STAI), respectively. Participants were followed for a median 5.9 years to track the incidence of CVD events (stroke, myocardial infarction, heart failure, and CVD-related mortality). We tested the BDI * STAI interaction effect in addition to the BDI and STAI main effects.
Results
Seventy-five women (15.3% of sample) experienced a CVD event, of which 18 were deaths attributed to cardiovascular causes. Results using Cox regression indicated a significant BDI * STAI interaction effect in the prediction of CVD events (p=.02) after covariate adjustment. Simple effect analyses indicated that depression scores were significant predictors of CVD events among women with low anxiety scores (HR=2.3 [in standard deviation units], 95% CI=1.3-3.9, p=.005) but not among women with higher levels of anxiety (HR=.99, 95% CI=.70-1.4, p=.95).
Conclusions
Among women with suspected myocardial ischemia, the value of depression symptoms for predicting CVD events varied by the severity of comorbid anxiety. These results suggest that the clinical utility of depression measures may be improved by using them in combination with measures of anxiety.
Keywords: Depression, anxiety, cardiovascular disease, women, prospective
Among psychiatric symptoms linked to cardiovascular disease (CVD), anxiety and depression are perhaps the most common. Both conditions are widespread among cardiac patients (1-3), each is associated with behavioral and pathophysiological markers of CVD risk (4-7), and the independence of anxiety and depression as predictors of CVD outcomes is supported by multiple studies (8-15). Anxiety and depressive disorders also share treatment approaches, with serotonin reuptake inhibitor medications, cognitive behavioral therapy, and their combination recognized as effective interventions for both conditions (16).
The high degree of overlap between anxiety and depression symptoms with regard to their co-morbidity in clinical populations, effective methods of treatment, and specific symptoms may represent more than coincidence. Some have suggested that anxiety and depression may be characteristics of a broader negative affect dimension (17-18). Others have argued that they are produced by the same dysfunctional biology or that they may originate from parallel genetic dispositions (19-21). Despite the strong convergence between these two psychiatric conditions and evidence of their independent value in predicting CVD risk, few studies have investigated the combined or interactive contribution of anxiety and depression in the prediction of CVD events (22-23). If anxiety and depression are indeed variations of a similar biopsychosocial origin, then either of two outcomes could follow: 1) These conditions could interchangeably predict CVD events, and the presence of both would not be more predictive than either alone; or 2) The co-occurrence of anxiety and depression could predict CVD risk above and beyond their independent predictions.
The literature provides differing views regarding these hypotheses. For one, depression is a more established predictor of cardiovascular outcomes than anxiety (13-14), the latter boasting a smaller and less consistent empirical literature to date (17). This observation appears to argue against treating the conditions interchangeably in regards to CVD risk, but comes with the limitation that much of the previous anxiety-CVD research has focused on men (e.g., 24). In contrast, recent findings drawn from large female cohorts such as the Nurse's Health Study (25) and Women's Health Initiative (26) have supported relationships between anxiety symptom severity and/or anxiety disorders with adverse cardiac events.
Two prospective studies to date have directly compared anxiety and depression alone versus jointly in predicting CVD outcomes (22-23). These studies produced evidence of independent statistical relationships between anxiety and depressive disorders with adverse cardiac events (22), and between questionnaire measures of phobic anxiety and depression and ventricular arrhythmias (23), respectively. Whereas Frasure-Smith (22) reported no added predictive effects of combining anxiety and depression status, Watkins and colleagues (23) also described the largest associations with ventricular arrhythmias as resulting from a composite of anxiety and depression scores. The fact that women comprised less than 30% of the sample in both studies could be important given the higher rates of anxiety and depressive disorders diagnosed in women relative to men (27).
This study describes relationships among anxiety, depression, and CVD mortality and events in a sample of women undergoing coronary angiography as part of an evaluation for suspected myocardial ischemia. We were able to expand upon previous research in this area using a large female cohort, followed for a median of nearly 6-years, in which we were able to directly control for CAD severity with the use of quantitative angiograms. Using questionnaire-measured severity of trait anxiety and depression symptoms, we specifically compared independent versus interactive prediction models.
Method
Participant recruitment and entrance criteria
Women were eligible for participation in the Women's Ischemia Syndrome Evaluation (WISE) study if they were older than 18 years and were referred by a physician for a coronary angiogram to evaluate suspected myocardial ischemia (28). The WISE study was designed to improve the diagnostic reliability of cardiovascular testing in women with suspected ischemic heart disease by focusing on symptomatic women whose CAD status was largely unknown. Exclusion criteria included major comorbidity compromising follow-up, pregnancy, contraindication to provocative diagnostic testing, cardiomyopathy, severe heart failure, recent myocardial infarction or revascularization procedures, significant valvular or congenital heart disease, and language barrier. All participants provided written informed consent, and IRB approval was obtained for all participating sites. Study enrollment was completed between 1996 and 2000, with follow-up, described below, carried out over a median of 5.9 years (25th percentile=2.5 years; 75th percentile=6.9 years) after study entry.
Measurement of CAD and Clinical Outcome Events
Quantitative analysis of coronary angiograms was performed at the WISE Angiographic Core Laboratory (Rhode Island Hospital, Providence, RI) by investigators blinded to all other subject data (29). Using the angiogram results, each participant was assigned a continuous coronary disease severity score based on a modified Gensini index (29). This severity score was developed with points assigned according to the category of severity of the stenosis (0-19, 20-49, 50-69, 70-89, 90-98, 99-100) adjusting for partial (any filling of the occluded vessel or its distal branches antegrade or retrograde via channels other than the original lumen) and complete collaterals. Scores were then adjusted according to lesion location with more proximal lesions receiving a higher weighting factor.
Women were contacted at six weeks post-baseline and annually thereafter to track their subsequent experiences of cardiovascular events and mortality. Follow-up consisted of a scripted telephone interview by an experienced nurse or physician. This data collection tool was validated previously against medical records (30). Non-fatal cardiovascular events included myocardial infarction (MI), congestive heart failure (CHF), and stroke. Death certificates were obtained and reviewed by a blinded study mortality committee to determine causes of death among deceased participants. In the current analyses, deaths coded as either probably or definitely resulting from cardiovascular causes were included as events.
Modifiable risk factors
Major CVD risk factors in the WISE protocol included smoking (defined as current, former, or never smokers), dyslipidemia, diabetes (insulin and non-insulin dependent), and hypertension. Risk factors were assessed by a combination of laboratory testing and physical exam (dyslipidemia, diabetes, hypertension) and self-report (smoking, physical inactivity). Patients also reported their highest level of education as a measure of socioeconomic status.
Depression and anxiety measures
Women completed the Beck Depression Inventory (BDI; 31) and the trait anxiety subscale from the State Trait Anxiety Inventory (STAI; 32) as measures of depressive and anxiety symptom severity, respectively. Each measure is validated as a predictor of objective health outcomes in cardiac populations (6). For descriptive purposes, we used BDI scores ≥ 10 to indicate the presence of at least mild depression symptoms (31). Women also reported use of antidepressants (medications taken for the treatment of depression) and anxiolytic medications in the week prior to study entry. We did not query mental health treatment details such as the medication type, length of treatment, efficacy, or dosage. In the present sample, internal consistency values for the BDI (Cronbach's α = .88) and STAI (Cronbach's α = .85) indicated acceptable levels of reliability, and scores on the two measures were highly correlated (r = .69, p < .001).
Statistical Analyses
Student t-tests (continuous variables) and chi-square analysis (categorical variables) were used to compare women with versus without BDI scores ≥ 10, and between women with versus without psychometric data on demographic and CVD risk factors. Relationships between BDI and STAI scores with study variables were expressed as correlation coefficients as an effect size measure (Pearson r coefficients between continuous measures, point-biserial r coefficients between continuous and dichotomous variables). A combined category indicating death from CVD causes or occurrence of one or more new CVD events (MI, stroke, and CHF) served as the primary outcome over follow-up. Associations between anxiety and depression with time to CVD death or outcomes were tested in Cox regression models after adjusting for covariates including age, ethnicity (Caucasian/Non-Caucasian), education history (≤ high school education), CVD risk factors, and CAD severity scores. Associations between continuous anxiety and depressive symptom severity from the BDI and STAI questionnaire scores were tested by entering each score into the model following the covariate terms and subsequently entering the anxiety x depression interaction term formed as a cross-product of the two questionnaire scores.
BDI and STAI scores were standardized (i.e., [score-mean]/standard deviation) before creating the cross-product interaction term to reduce multicollinearity risk (33), and the standardized forms of the questionnaires were used for the interpretation of main effects and interactions. The latter interaction method maximizes statistical power by using the full distribution of the questionnaire scores and is consistent with statistical guidelines demonstrating the flaws inherent in testing interactions based on artificially dichotomized scores (34). In the case of a significant interaction, we performed simple effect analyses comprised of models testing depression effects across low and high anxiety scores based on a median split of the STAI variable.
Plotted interaction values using continuous BDI and STAI scores were created using specialized interaction software (http://www.danielsoper.com/Interaction) and calculated using final model statistics (i.e., covariate-adjusted regression coefficients) in which all covariates and predictors were initially standardized (using same formula as above) before modeling. Because of variable skewing, CAD severity scores were log transformed for analyses. All statistical analyses were completed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA). For purposes of comparing our results with a previous study combining anxiety and depression questionnaires scores (23), we created a composite score by standardizing the BDI and STAI scores (i.e., converting the scores to a mean of zero and a standard deviation of one) and summing them.
Results
From the 936 women enrolled in WISE, 489 had complete data on psychosocial questionnaires, follow-up, and covariates used in the analyses. Because psychological questionnaires were phased in over the first year of the WISE protocol more than 400 women among the initial cohort did not complete the STAI, BDI or both. During preliminary analyses, WISE participants with and without psychosocial data were compared on a variety of demographic variables, CVD risk factor prevalence, and CAD severity, with the singular difference appearing that women without psychometric data had more severe CAD based on their CAD severity scores (means 16.8[16.5] versus 13.4[13.1), respectively, p<.05). Comparisons of the participating WISE sample, broken down by BDI scores, are shown in Table 1.
Table 1.
Demographic and CVD risk factors categorized by mental health status.
| Variable | BDI≥10 (N=217) |
BDI<10 (N=272) |
|---|---|---|
| Age (mean[SD]) | 55.7(11.0) | 59.3(11.3) |
| high school educated (%[n]) | 77%(167) | 85% (231)* |
| Non-Caucasian (%[n]) | 21 (46)% | 14%(38)* |
| BDI score (mean[SD]) | 17.6(7.4) | 4.7(2.7)* |
| STAI score (mean[SD]) | 22.6(5.5) | 15.9(4.0)* |
| CAD severity score (mean[SD]) | 12.7(12.2) | 14.0(13.8) |
| Current smoker (%[n]) | 26% (56) | 11% (30)* |
| Diabetic (%[n]) | 26% (56) | 21% (57) |
| Dyslipidemia (%[n]) | 67%(145) | 61%(166) |
| Hypertensive (%[n]) | 61%(132) | 56%(152) |
| CVD death (%[n]) | 6%(13) | 2%(5) |
| Total events†(%[n]) | 19.8%(43) | 11.8%(32)* |
| Using antidepressants (%[n]) | 29%(63) | 12%*(33) |
| Using anxiolytics (%[n]) | 25%(54) | 17%*(46) |
BDI groups differ, p<.05 BDI=Beck Depression Inventory CAD=Coronary artery disease CVD=Cardiovascular Disease STAI=State Trait Anxiety Inventory SD=Standard deviation CVD=Cardiovascular disease
Includes congestive heart failure, myocardial infarction, stroke, and cardiovascular deaths
BDI and STAI scores were associated with demographic variables, CVD risk factors, and CVD events in a parallel fashion at the bivariate level. Both BDI and STAI scores were associated with younger age (r's=-.16, -.18, respectively, p's<.01), higher rates of smoking (r's=.23, .24, respectively, p's<.001), greater use of antidepressants (r's=.26, .19, p's<.001, respectively) and anxiolytics (r's=.19, .16, p's<.001), and a greater likelihood of suffering a CVD event (i.e., congestive heart failure, MI, or stroke; r's=.15, .13, respectively, p's<.01). From the pool of 489 participants with complete data, 44.3% had BDI scores ≥ 10. Over a median 5.9 years of follow-up, 75 women (15.3% of sample) experienced at least one CVD outcome, of which 18 were deaths attributed to cardiovascular causes (3.6%).
Prediction of CVD death and events
In unadjusted Cox regression analyses run separately for each questionnaire, BDI scores (HR=1.27, 95% CI=1.06-1.51 in standard deviation units), but not STAI scores (HR=1.13, 95% CI=.92-1.4), were associated with combined CVD events and mortality. Forcing both questionnaire scores into the model with no additional covariates showed again that BDI scores (HR=1.5, 95% CI=1.1-2.0) but not STAI scores (HR=.83, 95% CI=.60-1.1) were associated with cardiovascular events. In contrast, use of the composite BDI and STAI score in place of the individual scale scores showed that this aggregate variable was not a significant predictor (HR=1.1, 95% CI=.99-1.2).
Results of a full model containing standardized covariate terms and a breakdown of BDI scores as predictors of CVD death and events at different levels of STAI scores are shown in Table 2. Despite the degree of statistical overlap in the two questionnaires (r=.69), collinearity diagnostics, including condition indices (range 1.0-3.1) and the Variance Inflation Factor (range 1.5-2.5), were well below values indicative of multicollinearity. CAD severity scores, current smoking status, completing less than a high school education, and a diagnosis of diabetes were independently significant predictors in the model. The BDI * STAI interaction was also significant (p=.02). The separate BDI values reported in Table 2 represent BDI-cardiovascular death and event relationships at STAI values below and above the median score of 24.0.
Table 2.
Results for anxiety and depression measures as predictors of cardiovascular death and events.
| Beck Depression Inventory (BDI) and State Trait Anxiety Inventory (STAI) scores. N=489. | |||
|---|---|---|---|
| Predictor | Hazard ratio | 95% CI | p-value |
| Age | .89 | .67-1.2 | .62 |
| Education | 1.3 | 1.1-1.7 | .03 |
| Race | 1.1 | .86-1.4 | .24 |
| CAD severity score | 1.8 | 1.4-2.3 | <.001 |
| Smoking status | 1.4 | 1.1-1.7 | .02 |
| Hypertensive | 1.3 | .98-1.7 | .06 |
| Dyslipidemia | 1.2 | .93-1.7 | .11 |
| Diabetic | 1.4 | 1.2-1.8 | <.001 |
| BDI score (at lower STAI scores based on median split) | 2.3 | 1.3-3.9 | .005 |
| BDI score (at higher STAI scores based on median split) | .99 | .70-1.4 | .95 |
Figure 1 illustrates the continuous interaction pattern, showing that BDI scores were increasingly effective as predictors of CVD death and events as STAI scores decreased. Simple slope values for the interaction lines plotted at the STAI mean (.06, 95% CI=.02-.11), and 1 standard deviation below the STAI mean (.10, 95% CI=.04-.16) differed significantly from zero, but the interaction slope at 1 standard deviation above the STAI mean (.02, 95% CI=-.01-.06) was nonsignificant. We repeated the latter analysis after further adjusting for participant's reported use of antidepressant and anxiolytic medications, but the results were unchanged.
Figure 1.
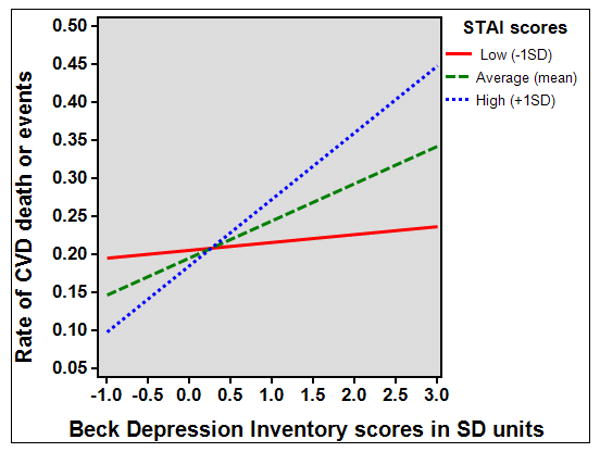
Relationship between Beck Depression Inventory scores and combined CVD death and event rates across three levels of State Trait Anxiety (STAI) Inventory scores (N=489). Depression was a significant predictor of CVD outcomes at low (n=93) and moderate levels of anxiety (n=313), but non-significant at higher anxiety levels (n=83).
Finally, Table 3 provides a breakdown of cardiovascular death and event totals among women classified by BDI and STAI standing. Using a 2×3 Analysis of Variance, the interaction effect was significant for this categorical model (F[2,482]=3.4, p=.03).
Table 3.
Cardiovascular death and event totals broken down by Beck Depression Inventory (BDI) and State Trait Anxiety Inventory (STAI) scores* (N=489).
| BDI ≥10 N=217 |
BDI<10 N=272 |
|
|---|---|---|
| Low anxiety (N=93) | 4 (36.3%) | 6 (7.3%) |
| Moderate anxiety (N=313) | 25 (19.5%) | 23 (12.7%) |
| High anxiety (N=83) | 14 (17.9%) | 3 (33.3%) |
Low, moderate, and high anxiety scores correspond to values ≤ 1 standard deviation below the mean, < than 1 standard deviation below the mean to < 1 standard deviation above the mean, and ≤ 1 standard deviation above the mean, respectively.
Discussion
Among women with suspected myocardial ischemia followed for a median 5.9 years, the joint use of anxiety and depression symptoms predicted incident CVD events and death above and beyond each symptom cluster. Using questionnaires to assess anxiety and depression symptom severity, the two mood dimension scores interacted such that a significant main effect relationship between depression and CVD death and events was found to vary across levels of anxiety. Specifically, the association between depression and CVD outcomes became progressively weaker across low, moderate, and high levels of anxiety symptoms. This result suggested that assessing depression and anxiety together more accurately predicted CVD outcomes than assessing either condition alone. Rates of elevated depression and anxiety were high in this sample, based on standards of either questionnaire scores or psychotropic use. However, these rates appeared appropriate given the symptomatic nature of the participants, along with the well-documented gender differences in rates of depression and anxiety documented in women (35). Depression and anxiety symptoms likewise overlapped substantially in this sample, with the inter-scale correlation approaching r=.70.
Anxiety and depressive disorders are common in the U.S. (35). The symptom overlap between these two mood conditions in cardiac populations is well-known (36-37), and many studies have examined both mood dimensions as predictors of cardiovascular outcomes (e.g., 2,4,7, 38-39). Nevertheless, depression rather than anxiety continues to garner the lion's share of attention in behavioral cardiology research. However, growing evidence suggests that anxiety warrants a greater focus in cardiac populations, particularly among women (40). Reports from cardiac samples, for example, have shown rates of anxiety disorders ranging from 25-36%, with rates twice as high among women compared to men (41-42). Prevalence statistics also show that rates of specific anxiety disorders such as agoraphobia, panic disorder, and generalized anxiety disorder are at least twice more common among women than men in medical and healthy samples (43-44). These findings parallel gender difference statistics for depression (43), and support the need for research addressing joint anxiety and depression effects in women.
Few studies have directly examined combined versus independent anxiety and depression symptoms as predictors of health outcomes in cardiovascular populations. Watkins (23) used questionnaire measures of depression (BDI) and phobic anxiety (Crown-Crisp index), with their results indicating that each symptom class was a predictor of ventricular arrhythmias alone, but when modeled together neither variable was significant. The latter effect was speculated to be a result of the high degree of overlap between the anxiety and depression scores. A composite (a sum of the standardized questionnaire scores) anxiety-depression score also predicted events in this study to a non-significantly greater degree than the separate anxiety and depression scores; however, no formal interaction between these measures was tested.
In patients with established coronary artery disease, Frasure-Smith and Lesperance (22) also assessed anxiety and depression comorbidity in the prediction of cardiac events using a combination of interview-based diagnoses and questionnaire scores (BDI-II and the anxiety subscale from the Hospital Anxiety and Depression Scale [HADS]). Interview-based diagnoses of generalized anxiety disorder and major depressive disorder were stronger predictors than questionnaire scores, but the combined presence of elevated anxiety and depression detected through diagnostic methods did not predict cardiovascular events more effectively than separate mood conditions. Interestingly, however, in a breakdown of questionnaire-derived depression effects across different levels of anxiety symptoms, these authors observed relatively stronger effects of depression when combined with low levels of anxiety. Although no specific test for an interaction effect was performed with the questionnaire scores, the latter pattern resembles the simple effect breakdown we have described here.
Our assessment of the joint effects of anxiety and depression indicated that higher anxiety levels attenuated (or, protected against, as the reader may prefer) the relationship between BDI scores and incident CVD death and events. BDI scores were significant predictors of CVD outcomes among women with moderate and low STAI scores, defined as those scoring at the scale mean and one standard deviation below the mean. However, among those with higher STAI scores, the BDI-CVD outcomes relationship was not significant. This effect is not due to confounding: to be a confounding relationship the confounding variable (in this case anxiety) must be statistically associated with both the predictor and the outcome (45), but anxiety was not significantly related to CVD outcomes in our analyses. Likewise, this effect cannot be explained by multicollinearity, as our diagnostic tests ruled out this possibility. Lacking evidence of a statistical artifact, the speculation that high levels of anxiety may in some way affect the accuracy or reliability of depression symptom reporting becomes more tenable. This effect may occur through the similarity in symptoms between these conditions (e.g., potentially artificially elevating depression scores through an overlap in somatic symptoms), via shared pathophysiological mechanisms (e.g., impaired heart rate variability or increased sympathetic nervous system activation), or behavioral correlates (e.g., anxious-depressed patients may more actively pursue healthcare, which might buffer the risk associated with depression). In regards to the latter speculation, depression was recently shown to predict increased healthcare usage in WISE participants (46). Finally, the fact that WISE participants were enrolled specifically because they had clinical symptoms serious enough to warrant coronary angiographic testing may also have influenced the anxiety-depression relationship, as depression and anxiety symptoms are known to correlate with cardiac symptom reporting (e.g., 6-7). Clearly, these topics will require further investigation to resolve.
The substantial overlap we observed between anxiety and depressive symptoms appears characteristic of cardiac samples. For example, Denollet and colleagues (36-38) reported that a mixed mood profile comprised of anxiety and depressive symptoms, rather than depression alone, was the most common presentation in post-MI patients (37). At least 90% of their post-MI patients meeting criteria for depression showed evidence of mixed anxiety-depression, leading the authors to conclude that anxiety is a common element of post-MI depression. The Frasure-Smith sample (22) experienced similar symptom overlap, from which 77.3% of patients with high BDI-II scores also had elevated HADS anxiety scores. Combined with our present findings, these collective results suggest that investigations reporting on depression alone in association with CVD outcomes are greatly underestimating the degree of comorbidity with anxiety symptoms. Whether this symptom overlap is more accurately conceptualized using composite scores (23), categorical breakdowns of anxiety and depression scores (22), factor analyses to create a mood meta-factor (17), or interaction terms as used in the current study, remains an empirical question.
In practice, one of the most difficult challenges facing a clinician is to accurately interpret the frequent overlap of depression, anxiety, and CVD symptoms. Are mental health symptoms a cause, consequence, or exacerbating agent in the patient's medical presentation? Alternatively, could both the mental health and CVD symptoms be the result of a common factor, such as inflammation? To add even more complexity, the role of mental health symptoms may change at different stages of the CVD process in a given patient, and the various roles are not exclusive. An example of this complexity is apparent in the interpretation of results by Walters and colleagues (47), who reported that newly diagnosed panic attacks or panic disorder were associated with an increased risk of MI or heart disease diagnosis in a sample of nearly 400,000 adults. Although panic symptoms were more strongly associated with cardiovascular outcomes in younger participants (<50 years of age), even in this higher risk group the authors point out that it cannot be clearly inferred whether panic symptoms represent a risk factor for cardiac outcomes or whether early stages of CVD were simply misdiagnosed as an anxiety condition.
How clinicians conceptualize this symptom overlap will go far in determining their diagnoses, treatment plans, and possible referrals for additional testing and services. At present, unfortunately, no foolproof guidelines for navigating this decision process exist, but evidence of errors and biases in current practices is abundant. Numerous studies, for example, indicate that women presenting with cardiac symptoms are less aggressively diagnosed and treated (e.g., 48-49), despite women having overall higher CVD death rates (50), suggesting that cardiologists are more likely to interpret women's symptoms as originating from non-cardiac or mental health sources.
Study limitations
This study offers some novel methodological features compared to previous research, including: (1) examining independent versus interacting anxiety-depression relationships with CVD events in the largest female cohort to date; (2) tracking CVD outcomes over nearly six years, roughly twice the length of previous efforts; (3) objectively quantifying the degree of CAD severity for use as a covariate, and; (4) examining trait anxiety rather than specific anxiety disorder subsets such as generalized anxiety disorder and phobic anxiety, which may create a different pattern of associations. However, several important limitations deserve acknowledgement. Our results addressed cardiovascular outcomes in a sample comprised exclusively of clinically symptomatic women, among whom obstructive CAD was present in approximately 40% (28), which differs substantially from the two previous investigations reported on this topic that included only participants with known CVD. The recruitment methods followed for WISE were intended to reflect the usual clinical circumstances of symptomatic women undergoing assessment for the presence of CAD; however, this study characteristic limits our ability to generalize findings to women with known CAD and asymptomatic samples. A substantial number of WISE participants did not complete the BDI and STAI questionnaires due to the absence of these measures from the baseline protocol during the initial months of the study. Psychological questionnaires were administered only at baseline, and no psychiatric interviews were included to document mental disorders. Although we found no evidence of bias between the groups completing versus not completing the mood questionnaires, we cannot rule out the possibility that the later cohort differed on unmeasured variables. Lastly, our results document a statistical interaction between depression and anxiety symptoms, which should not be confused with a biological interaction described in some epidemiological research. In contrast to biological interactions, which describe an effect emerging from the combination of two or more causal factors, statistical interactions carry the limitations of a non-causal interpretation and the effects being dependent on the choice of measurement scale (51).
Summary
Among patients with CVD, both anxiety and depression are common and these symptom dimensions overlap substantially. However, the cardiovascular research literature to date has largely examined these mood dimensions as separate predictors despite their known congruence. In a sample of women undergoing coronary angiography for the assessment of suspected myocardial ischemia, we specifically addressed the combination of anxiety and depression symptoms, observing that the interaction between anxiety and depression measures was superior in the prediction of CVD death and events compared to separate use of the mood measures. Perhaps our most provocative finding was that elevated anxiety appeared to diminish the effectiveness of depression as a predictor. The high rate of anxiety and depression co-occurrence documented both in this study and in previous research, combined with the value observed here for the joint value of these emotional dimensions as CVD event predictors, presents a compelling argument for moving beyond single construct research in behavioral cardiology and more towards theoretical models that incorporate multiple dimensions of negative affect.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, a GCRC grant MO1-RR00425 from the National Center for Research Resources (B. Delia Johnson, Vera Bittner, Carl J. Pepine, Diane A. Vido, and C. Noel Bairey Merz are site representatives for the aforementioned funding sources), and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey (C. Noel Bairey Merz), the Women's Guild of Cedars-Sinai Medical Center, Los Angeles, California (C. Noel Bairey Merz), the Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania (B. Delia Johnson, Diane A. Vido) the Edythe Broad Women's Heart Research Endowment, Cedars-Sinai Medical Center, Los Angeles, California (C. Noel Bairey Merz), and the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, California (C. Noel Bairey Merz).
Abbreviations
- WISE
Women's Ischemia Syndrome Evaluation
- CVD
Cardiovascular disease
- CAD
Coronary artery disease
- MDD
Major Depressive Disorder
- GAD
Generalized anxiety disorder
- CHF
Congestive heart failure
- MI
Myocardial infarction
- BDI
Beck Depression Inventory
- STAI
State Trait Anxiety Inventory
- HADS
Hospital Anxiety and Depression Scale
References
- 1.Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30:208–25. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Fan AZ, Strine TW, Jiles R, Mokdad AH. Depression and anxiety associated with cardiovascular disease among persons aged 45 years and older in 38 states of the United States, 2006. Prev Med. 2008;46:445–50. doi: 10.1016/j.ypmed.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Herbst S, Pietrzak RH, Wagner J, White WB, Petry NM. Lifetime major depression is associated with coronary heart disease in older adults: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2007;69:729–34. doi: 10.1097/PSY.0b013e3181574977. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Freedland KE, Stein PK. Anxiety, depression, and heart rate variability. Psychosomatic medicine. 2000;62:84–7. doi: 10.1097/00006842-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Aschbacher K, Mills PJ, von Känel R, Hong S, Mausbach BT, Roepke SK, Dimsdale JE, Patterson TL, Ziegler MG, Ancoli-Israel S, Grant I. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun. 2008;22:493–502. doi: 10.1016/j.bbi.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 7.Rothenbacher D, Hahmann H, Wüsten B, Koenig W, Brenner H. Symptoms of anxiety and depression in patients with stable coronary heart disease: prognostic value and consideration of pathogenetic links. Eur J Cardiovasc Prev Rehabil. 2007;14:547–54. doi: 10.1097/HJR.0b013e3280142a02. [DOI] [PubMed] [Google Scholar]
- 8.Shen BJ, Avivi YE, Todaro JF, Spiro A, 3rd, Laurenceau JP, Ward KD, Niaura R. Anxiety characteristics independently and prospectively predict myocardial infarction in men the unique contribution of anxiety among psychological factors. J Am Coll Cardiol. 2008;51:113–9. doi: 10.1016/j.jacc.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Shibeshi WA, Young-Xu Y, Blatt CM. Anxiety worsens prognosis in patients with coronary artery disease. Journal of the American College of Cardiology. 2007;49:2021–7. doi: 10.1016/j.jacc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Székely A, Balog P, Benkö E, Breuer T, Székely J, Kertai MD, Horkay F, Kopp MS, Thayer JF. Anxiety predicts mortality and morbidity after coronary artery and valve surgery--a 4-year follow-up study. Psychosom Med. 2007;69:625–31. doi: 10.1097/PSY.0b013e31814b8c0f. [DOI] [PubMed] [Google Scholar]
- 11.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease: the normative aging study. Circulation. 1994;90:2225–9. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- 12.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 13.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 14.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 15.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O'Connor CM, Adams KF, Jr, Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167:367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, den Boer JA, Fineberg NA, Knapp M, Scott J, Wittchen HU. British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005 Nov;19:567–96. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- 17.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 18.Bleil ME, Gianaros PJ, Jennings JR, Flory JD, Manuck SB. Trait negative affect: toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosom Med. 2008 Apr;70(3):328–37. doi: 10.1097/PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- 19.Hettema JM. What is the genetic relationship between anxiety and depression. Am J Med Genet C Semin Med Genet. 2008 May 15;148(2):140–6. doi: 10.1002/ajmg.c.30171. [DOI] [PubMed] [Google Scholar]
- 20.Flory JD, Manuck SB, Matthews KA, Muldoon MF. Serotonergic function in the central nervous system is associated with daily ratings of positive mood. Psychiatry Res. 2004 Nov 30;129(1):11–9. doi: 10.1016/j.psychres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Sibille E, Hen R. Combining genetic and genomic approaches to study mood disorders. Eur Neuropsychopharmacol. 2001 Dec;11(6):413–21. doi: 10.1016/s0924-977x(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 22.Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 23.Watkins LL, Blumenthal JA, Davidson JR, Babyak MA, McCants CB, Jr, Sketch MH., Jr Phobic anxiety, depression, and risk of ventricular arrhythmias in patients with coronary heart disease. Psychosom Med. 2006;68:651–6. doi: 10.1097/01.psy.0000228342.53606.b3. [DOI] [PubMed] [Google Scholar]
- 24.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation. 1994 Nov;90(5):2225–9. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- 25.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 26.Smoller JW, Pollack MH, Wassertheil-Smoller S, Jackson RD, Oberman A, Wong ND, Sheps D. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women's Health Initiative Observational Study. Arch Gen Psychiatry. 2007 Oct;64(10):1153–60. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington D.C: American Psychological Association; 1994. [Google Scholar]
- 28.Bairey Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology, and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 29.Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Bairey Merz CN. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] study angiographic core laboratory) 2001;87:937–941. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, Robertson DH, Alexander C, Nag S, Cook JR, Demopoulos LA, DiBattiste PM, Cannon CP, Weintraub WS. Cost and cost effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2002;288:1851–1858. doi: 10.1001/jama.288.15.1851. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT. Depression inventory. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- 32.Spielberger CD, Gorsuch RC, Lushene RE. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 33.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, London: Sage; 1991. [Google Scholar]
- 34.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 35.Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137–58. doi: 10.1146/annurev.clinpsy.3.022806.091444. [DOI] [PubMed] [Google Scholar]
- 36.Martens EJ, Smith OR, Denollet J. Psychological symptom clusters, psychiatric comorbidity and poor self-reported health status following myocardial infarction. Ann Behav Med. 2007;34:87–94. doi: 10.1007/BF02879924. [DOI] [PubMed] [Google Scholar]
- 37.Denollet J, Strik JJ, Lousberg R, Honig A. Recognizing increased risk of depressive comorbidity after myocardial infarction: looking for 4 symptoms of anxiety-depression. Psychother Psychosom. 2006;75:346–52. doi: 10.1159/000095440. [DOI] [PubMed] [Google Scholar]
- 38.Strik JJ, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. J Am Coll Cardiol. 2003;42:1801–7. doi: 10.1016/j.jacc.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Pignay-Demaria V, Lesperance F, Demaria RG, Frasure-Smith N, Perrault LP. Depression and anxiety and outcomes of coronary artery bypass surgery. The Annals of thoracic surgery. 2003;75:314–21. doi: 10.1016/s0003-4975(02)04391-6. [DOI] [PubMed] [Google Scholar]
- 40.Kubzansky LD, Kawachi I, Weiss ST, Sparrow D. Anxiety and coronary heart disease: a synthesis of epidemiological, psychological, and experimental evidence. Ann Behav Med. 1998;20:47–58. doi: 10.1007/BF02884448. [DOI] [PubMed] [Google Scholar]
- 41.Crowe JM, Runions J, Ebbesen LS, Oldridge NB, Streiner DL. Anxiety and depression after acute myocardial infarction. Heart Lung. 1996;25:98–107. doi: 10.1016/s0147-9563(96)80111-7. [DOI] [PubMed] [Google Scholar]
- 42.Todaro JF, Shen BJ, Raffa SD, Tilkemeier PL, Niaura R. Prevalence of anxiety disorders in men and women with established coronary heart disease. J Cardiopulm Rehabil Prev. 2007;27:86–91. doi: 10.1097/01.HCR.0000265036.24157.e7. [DOI] [PubMed] [Google Scholar]
- 43.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 44.Magee WJ, Eaton WW, Wittchen HU, McGonagle KA, Kessler RC. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry. 1996;53:159–168. doi: 10.1001/archpsyc.1996.01830020077009. [DOI] [PubMed] [Google Scholar]
- 45.Judea Pearl. Department of Statistics Papers; Jan 30, 1998. Why There Is No Statistical Test for Confounding, Why Many Think There Is, and Why They Are Almost Right. Paper 1998013001 http://repositories.cdlib.org/uclastat/papers/1998013001. [Google Scholar]
- 46.Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, Cornell CE, Eteiba W, Sheps DS, Francis J, Krantz DS, Bairey Merz CN, Parashar S, Handberg E, Vido DA, Shaw LJ. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women's Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53:176–83. doi: 10.1016/j.jacc.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walters K, Rait G, Petersen I, Williams R, Nazareth I. Panic disorder and risk of new onset coronary heart disease, acute myocardial infarction, and cardiac mortality: cohort study using the general practice research database. Eur Heart J. 2008;29:2981–8. doi: 10.1093/eurheartj/ehn477. [DOI] [PubMed] [Google Scholar]
- 48.Shaw LJ, Miller DD, Romeis JC, Kargl D, Younis LT, Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559–66. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 49.Epstein AM, Weissman JS, Schneider EC, Gatsonis C, Leape LL, Piana RN. Race and gender disparities in rates of cardiac revascularization: do they reflect appropriate use of procedures or problems in quality of care? Med Care. 2003;41:1240–55. doi: 10.1097/01.MLR.0000093423.38746.8C. [DOI] [PubMed] [Google Scholar]
- 50.American Heart Association. Heart Disease and Stroke Statistics. [January 15, 2004];2004 Update. Available at: http://americanheart.org/downloadable/heart/1072969766940HSStats2004Update.pdf.
- 51.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–9. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]


