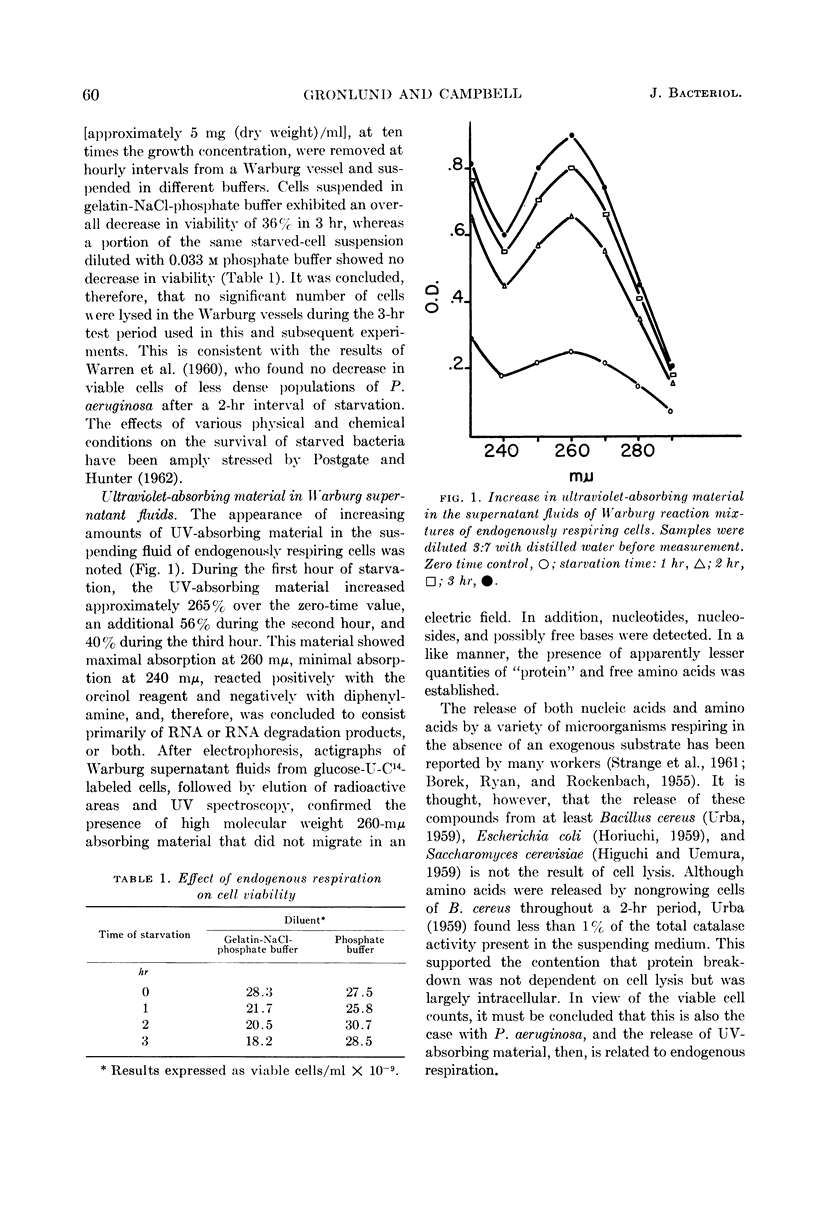
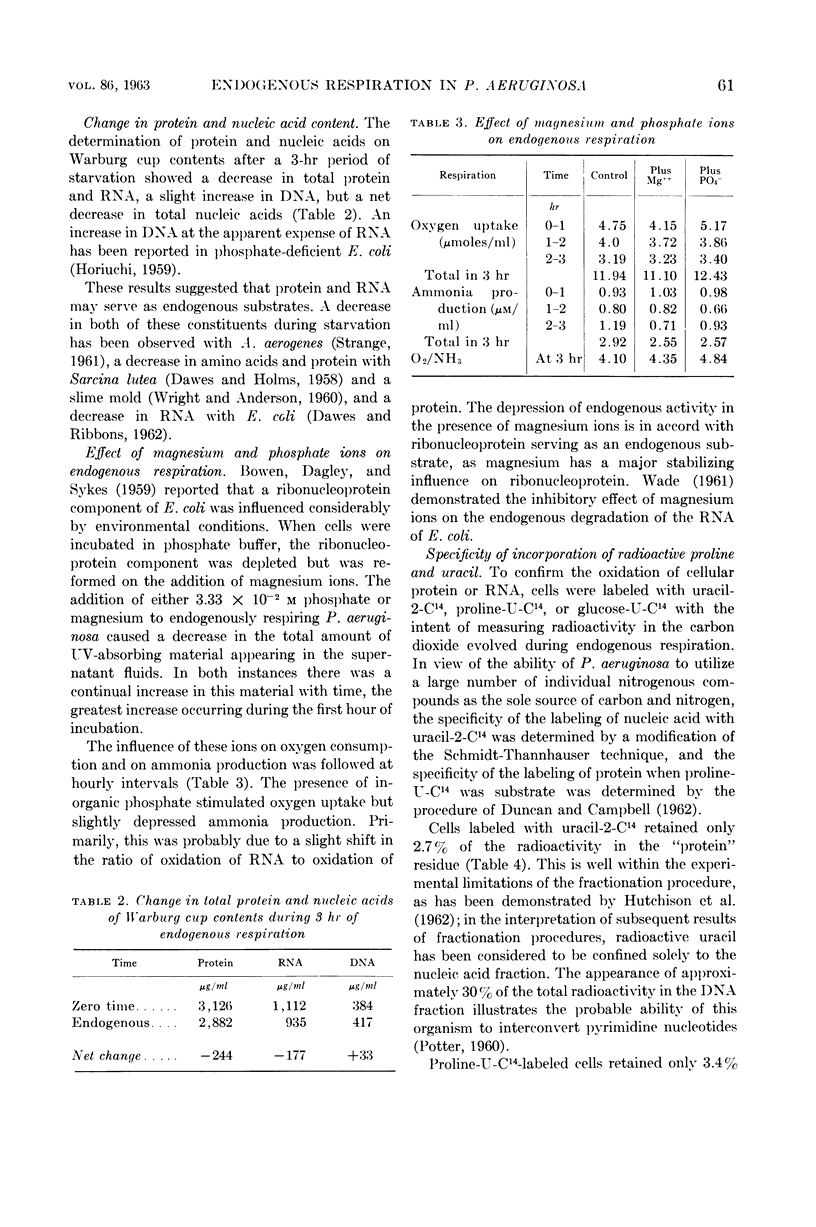
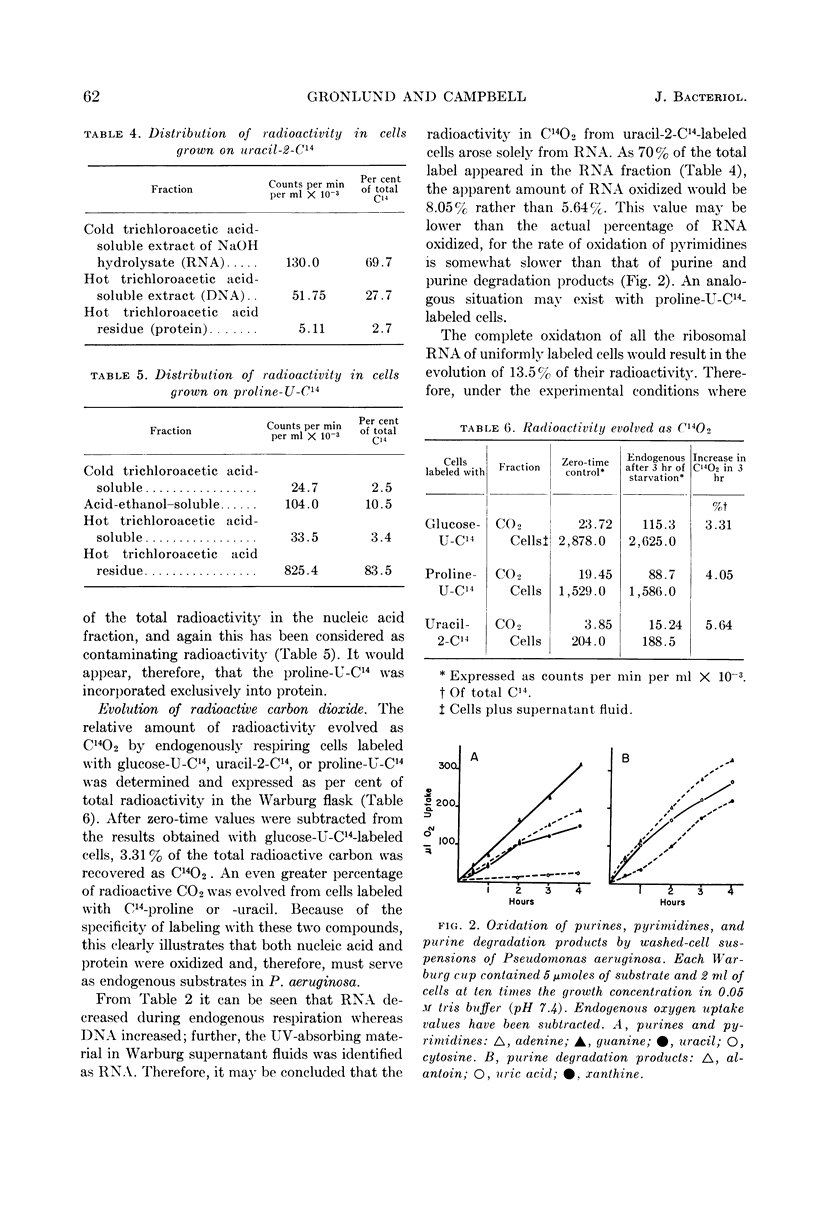
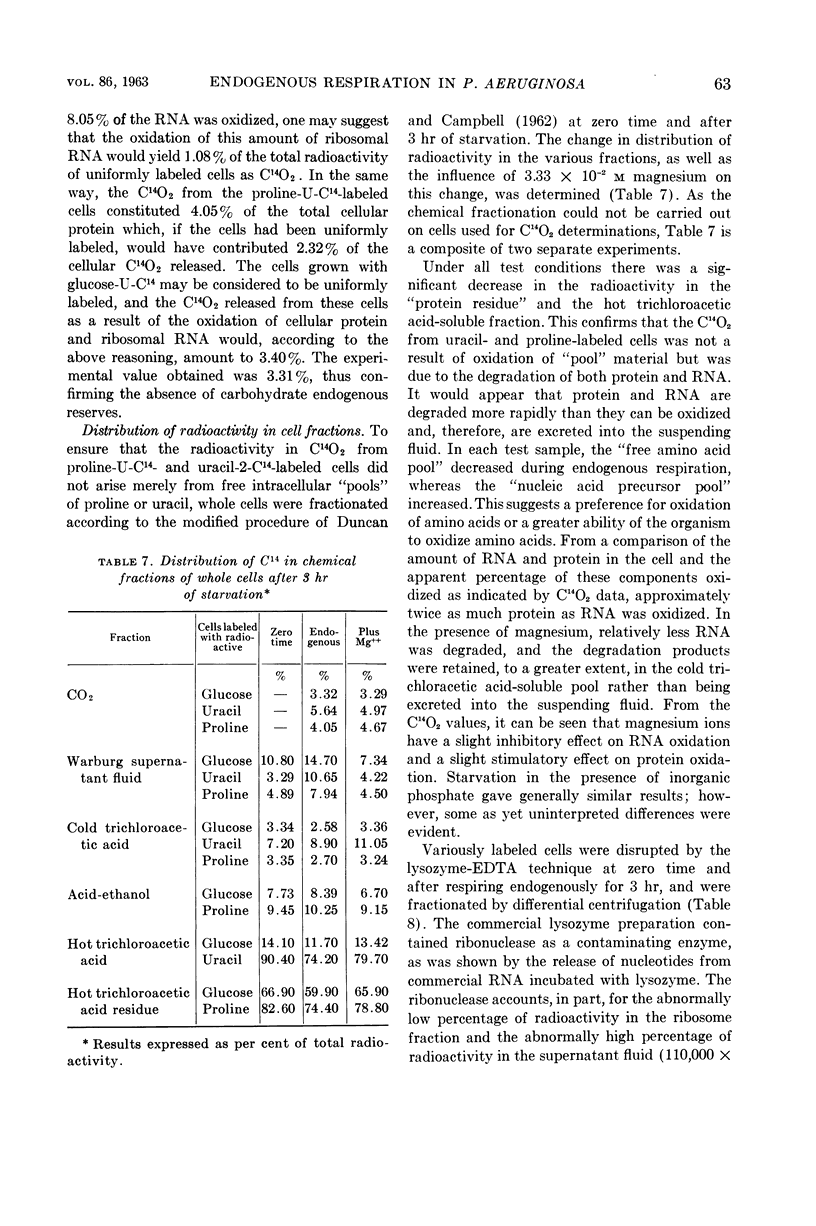
Abstract
Gronlund, Audrey F. (University of British Columbia, Vancouver, Canada) and J. J. R. Campbell. Nitrogenous substrates of endogenous respiration in Pseudomonas aeruginosa. J. Bacteriol. 86:58–66. 1963.—The nature of the nitrogenous reserves of Pseudomonas aeruginosa that are oxidized during endogenous respiration was studied by following the changes in the chemical constituents and in the distribution of radioactivity of starving cells that had been grown on C14-labeled substrates. The total protein and nucleic acid of Warburg vessel contents decreased during starvation. Deoxyribonucleic acid increased slightly, whereas ribonucleic acid (RNA) decreased. C14O2 was evolved from endogenously respiring cells specifically labeled in the nucleic acid fraction and from cells specifically labeled in the protein fraction. Chemical fractionation of C14-labeled cells showed a decrease in hot trichloroacetic acid-soluble and -insoluble compounds, indicating that the C14O2 arose from the degradation of RNA and protein and not free pool compounds. A decrease in ribosomal RNA and protein was evident from physical fractionations of starved, labeled cells. An enzyme responsible for the initiation of ribosomal degradation was found to be associated with the ribosome fraction. It was concluded that oxidation of the ribonucleo-protein during endogenous respiration may be a general phenomenon in microorganisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOREK E., RYAN A., ROCKENBACH J. Nucleic acid metabolism in relation to the lysogenic phenomenon. J Bacteriol. 1955 Apr;69(4):460–467. doi: 10.1128/jb.69.4.460-467.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN T. J., DAGLEY S., SYKES J. A ribonucleoprotein component of Escherichia coli. Biochem J. 1959 Jul;72:419–425. doi: 10.1042/bj0720419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL J. J., GRONLUND A. F., DUNCAN M. G. Endogenous metabolism of Pseudomonas. Ann N Y Acad Sci. 1963 Jan 21;102:669–677. doi: 10.1111/j.1749-6632.1963.tb13667.x. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., HOLMS W. H. Metabolism of Sarcina lutea. III. Endogenous metabolism. Biochim Biophys Acta. 1958 Nov;30(2):278–293. doi: 10.1016/0006-3002(58)90052-0. [DOI] [PubMed] [Google Scholar]
- DOUDOROFF M., STANIER R. Y. Role of poly-beta-hydroxybutyric acid in the assimilation of organic carbon by bacteria. Nature. 1959 May 23;183(4673):1440–1442. doi: 10.1038/1831440a0. [DOI] [PubMed] [Google Scholar]
- Duncan M. G., Campbell J. J. OXIDATIVE ASSIMILATION OF GLUCOSE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1962 Oct;84(4):784–792. doi: 10.1128/jb.84.4.784-792.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRONLUND A. F., CAMPBELL J. J. Nitrogenous compounds as substrates for endogenous respiration in microorganisms. J Bacteriol. 1961 May;81:721–724. doi: 10.1128/jb.81.5.721-724.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI M., UEMURA T. Release of nucleotides from yeast cells. Nature. 1959 Oct 31;184:1381–1383. doi: 10.1038/1841381a0. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- HUTCHISON W. C., DOWNIE E. D., MUNRO H. N. Factors affecting the Schneider procedure for estimation of nucleic acids. Biochim Biophys Acta. 1962 May 14;55:561–570. doi: 10.1016/0006-3002(62)90835-1. [DOI] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. The relation of ribosome content to the rate of enzyme synthesis in Aerobacter aerogenes. Biochim Biophys Acta. 1962 Jan 22;55:139–151. doi: 10.1016/0006-3002(62)90940-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E. Induced enzyme synthesis in aqueous suspensions of starved stationary phase Aerobacter aerogenes. Nature. 1961 Sep 23;191:1272–1273. doi: 10.1038/1911272a0. [DOI] [PubMed] [Google Scholar]
- STRASDINE G. A., HOGG L. A., CAMPBELL J. J. A ribosomal polynucleotide phosphorylase in Pseudomonas aeruginosa. Biochim Biophys Acta. 1962 Jan 22;55:231–232. doi: 10.1016/0006-3002(62)90956-3. [DOI] [PubMed] [Google Scholar]
- URBA R. C. Protein breakdown in Bacillus cereus. Biochem J. 1959 Mar;71(3):513–518. doi: 10.1042/bj0710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE H. E., LOVETT S. Polynucleotide phosphorylase in ribosomes from Escherichia coli. Biochem J. 1961 Nov;81:319–328. doi: 10.1042/bj0810319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE H. E. The autodegradation of ribonucleoprotein in Escherichia coli. Biochem J. 1961 Mar;78:457–472. doi: 10.1042/bj0780457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN R. A., ELLS A. F., CAMPBELL J. J. Endogenous respiration of Pseudomonas aeruginosa. J Bacteriol. 1960 Jun;79:875–879. doi: 10.1128/jb.79.6.875-879.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT B. E., ANDERSON M. L. Protein and amino acid turnover during differentiation in the slime mold. I. Utilization of endogenous amino acids and proteins. Biochim Biophys Acta. 1960 Sep 9;43:62–66. doi: 10.1016/0006-3002(60)90407-8. [DOI] [PubMed] [Google Scholar]


