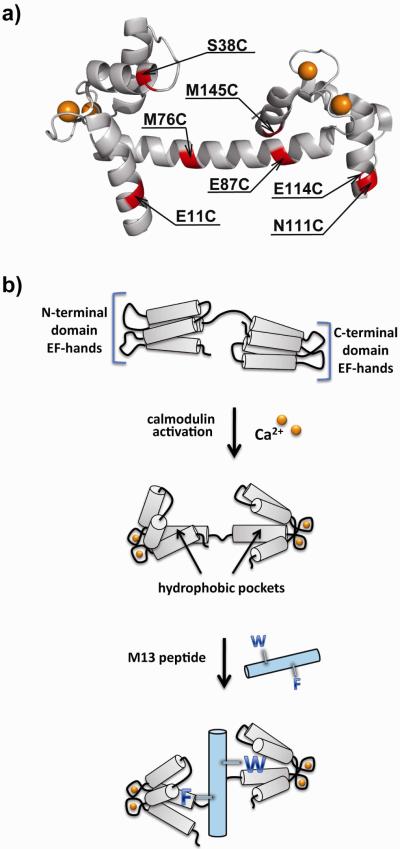
Figure 1.
(a) Crystal structure of the Ca2+-CaM complex deposited by Rupp et. al. (PDB entry 1UP5) (34). The structure indicates the seven sites selected for cysteine mutagenesis (red). The bound calcium ions are indicated by spheres (yellow). (b) Ca2+ activation of a CaM mutant (grey) can result in the burying of an attached solvatochromic fluorophore within one of the newly formed hydrophobic pockets. In response, the fluorophore exhibits a change in one or more of the emission properties. Subsequent addition of the M13 peptide (cyan) can displace the fluorophore from the pocket through insertion of an aromatic side-chain from the phenylalanine or tryptophan residue indicated here using the single-letter notation for amino acids.