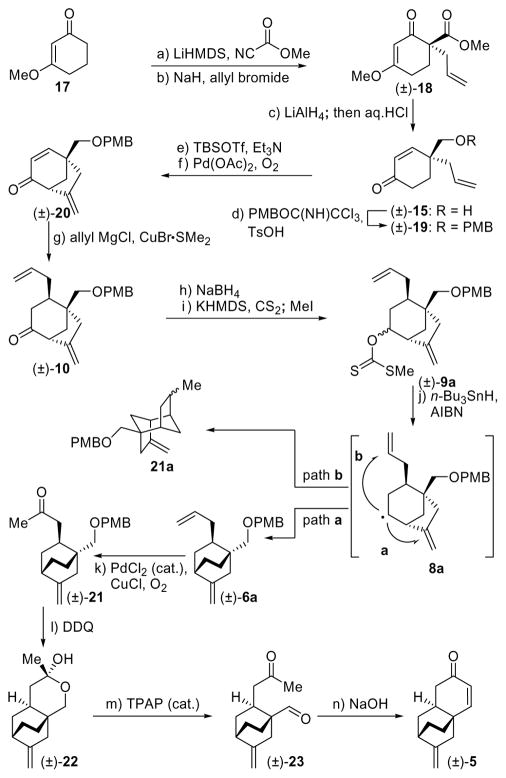
Scheme 1.
Synthesis of Racemic Tricyclic Enone [(±)]-5]a
aReagents and conditions: a) LiHMDS (1.0 M in THF, 1.2 equiv), methyl cyanoformate (1.2 equiv), THF, −78 → 0 °C, 2 h, 92%; b) NaH (1.5 equiv), allyl bromide (1.5 equiv), THF, 0 → 25 °C, 2 h, 79%; c) LiAlH4 (1.0 M in THF, 1.2 equiv), Et2O, 0 → 25 °C, 3 h; then HCl (2 M in MeOH, 6.0 equiv), 25 °C, 16 h, 82%; d) PMBOC(NH)CCl3 (2.0 equiv), TsOH (0.1 equiv), CH2Cl2, 0 → 25 °C, 2.5 h, 85%; e) TBSOTf (2.5 equiv), Et3N (4.0 equiv), THF, 0 °C, 1 h, 91%; f) Pd(OAc)2 (0.1 equiv), O2 (balloon), DMSO, 45 °C, 12 h, 85%; g) CuBr•Me2S (2.0 equiv), allyl magnesium chloride (1.7 M in THF, 4.0 equiv), THF, −78 → −40 °C, 2 h, 86%; h) NaBH4 (2.5 equiv), MeOH, −5 → 25 °C, 30 min, 97%; i) KHMDS (0.5 M in toluene, 5.0 equiv), CS2 (10.0 equiv), MeI (10.0 equiv), THF, −78 → 25 °C, 2.5 h, 92%; j) n-Bu3SnH (2.0 equiv), AIBN (0.08 equiv), toluene, 110 °C, 8 h, 92%; k) PdCl2 (0.25 equiv), CuCl (1.5 equiv), O2 (balloon), DMF:H2O (6.6:1), 25 °C, 24 h, 81%; l) DDQ (1.2 equiv), CH2Cl2:H2O 20:1, 25 °C, 1 h, 53%; m) TPAP (0.03 equiv), NMO (6.5 equiv), CH2Cl2, 25 °C, 4 h, 54%; n) NaOH (6.0 equiv), EtOH, 25 °C, 8 h, 99%.