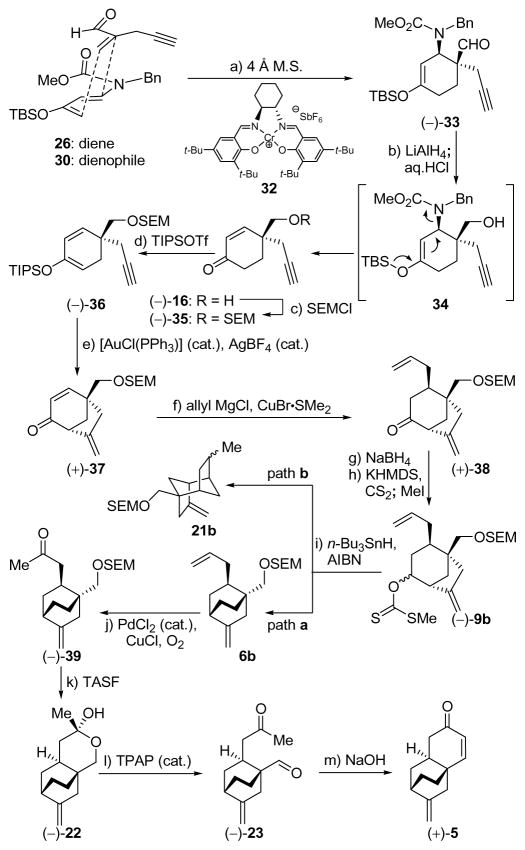
Scheme 3.
First Generation Catalytic Asymmetric Synthesis of Tricyclic Enone [(+)-5]a
aReagents and conditions: a) 30 (1.0 equiv), 26 (1.7 equiv), 32 (0.05 equiv), 4 Å M.S., CH2Cl2, −60 °C, 60 h, 92%; b) LiAlH4 (1.0 M in THF, 1.5 equiv), Et2O, −78 → −40 °C, 2 h; then HCl (2 M in MeOH, 10.0 equiv), 25 °C, 16 h, 63%; c) SEMCl (1.2 equiv), Et3N (4.0 equiv), 4-DMAP (0.1 equiv), THF, 70 °C, 16 h, 94%; d) TIPSOTf (1.5 equiv), Et3N (3.0 equiv), −78 → 0 °C, 1 h, 97%; e) [AuCl(PPh3)] (0.02 equiv), AgBF4 (0.02 equiv), toluene/MeOH (10:1), 25 °C, 30 min, 94%; f) allyl magnesium chloride (1.7 M in THF, 4.0 equiv), CuBr•Me2S (2.0 equiv), THF, −78 °C, 1.5 h, 74%; g) NaBH4 (2.5 equiv), MeOH, −5 → 25 °C, 1 h, 97%; h) CS2 (10.0 equiv), KHMDS (0.5 M in toluene, 5.0 equiv), MeI (5.0 equiv), THF, −78 → 25 °C, 2.5 h, 100%; i) n-Bu3SnH (2.0 equiv), AIBN (0.08 equiv), toluene, 100 °C, 20 min, 92%; j) PdCl2 (0.25 equiv), CuCl (1.5 equiv), O2 (balloon), DMF/H2O (6.6:1), 25 °C, 24 h, 50% (2 steps); k) TASF (10.0 equiv), DMPU, 80 °C, 1.5 h, 63% (84% based on 25% recovered starting material); l) TPAP (0.03 equiv), NMO (6.5 equiv), CH2Cl2, 25 °C, 4 h, 54%; m) NaOH (6.0 equiv), EtOH, 25 °C, 8 h, 99%.