Table 1.
Optimization of the Catalytic Asymmetric Diels–Alder Reactiona
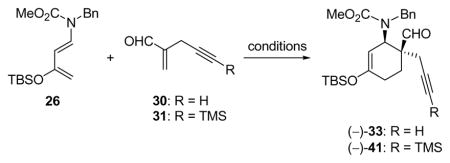 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Dienophile | Diene (equiv) | Catalyst (mol %) | Temp. (°C) | Time (h) | Yield (%) | ee (%) |
| 1 | 30 | 1.5 | 32 (5) | 25 | 60 | 85 | 75 |
| 2 | 30 | 1.5 | 32 (5) | −40 | 60 | 87 | 85 |
| 3 | 30 | 2.0 | 32 (5) | −60 | 60 | 92 | 93b |
| 4 | 30 | 1.8 | 40 (5) | 0 | 2 | 79 | 76 |
| 5 | 31 | 1.8 | 40 (5) | 0 | 1.5 | 92 | 93 |
| 6 | 31 | 1.5 | 40 (1) | 0 | 0.5 | 93 | 94 |
| 7 | 31 | 1.1 | 40 (0.1) | 0 | 0.5 | 97 | 96 |
| 8 | 31 | 1.5 | none | 25 | 0.5 | 5 | 0 |
Reactions were carried out on 0.1–1.0 mmol scale with 1.0 equiv. dienophile 30 or 31 in the presence of 4 Å M. S. in CH2Cl2.
Enantiomeric excess (ee) ranged from 86–93%.
