Table 3.
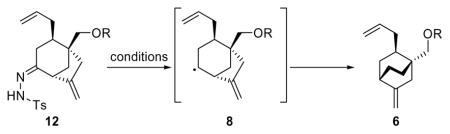
Optimization of the Radical-Based Conversion of Hydrazones 12 to [2.2.2] Bicyclo Systems 6a
 | |||||
|---|---|---|---|---|---|
| Entry | R (compound) | Reductant (equiv) | Solvent | Temp. (°C) | Yield (%) |
| 1 | MOM (12a) | NaBH3CN (3.8)b | THF | 80 | 0c |
| 2 | PMB (12b) | NaBH3CN (3.8)b | THF | 80 | 0c |
| 3 | Piv (12c) | NaBH3CN (3.8)b | THF | 80 | 0c |
| 4 | SEM (12d) | NaBH4 (1.4) | TiHF | 90 | 0c |
| 5 | SEM (12d) | NaBH(OAc)3 (4.0) | DCE:AcOH (9:1) | 100 | 0d |
| 6 | SEM (12d) | NaBH4 (8.0) | CH2Cl2:MeOH (1:1) | 45 | 50 |
| 7 | SEM (12d) | NaBH4 (8.0) | THF:MeOH (1:1) | 70 | 47 |
| 8 | SEM (12d) | NaBH4 (8.0) | CH2Cl2:MeOH (1:1) | 25 | 0c |
| 9 | SEM (12d) | NaBH4 (8.0) | CH2Cl2:MeOH (20:1) | 45 | 55 |
| 10 | SEM (12d) | NaBH4 (8.0) | CH2Cl2:EtOH (20:1) | 45 | 60 |
| 11 | SEM (12d) | NaBH4 (2.0 × 3) | CH2Cl2:MeOH (20:1) | 45 | 70 |
| 12 | H (12) | NaBH4 (2.0 × 4) | CH2Cl2:MeOH (20:1) | 45 | 74 (6f) |
Reactions were carried out on 0.1–1.0 mmol scale.
With 3.8 equiv. ZnCl2.
Only decomposition observed.
No reaction observed.
