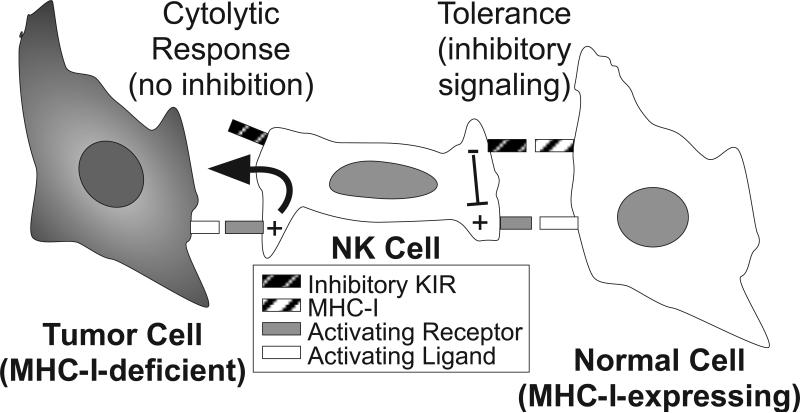
Figure 3. Surface receptor interactions establishing the basis for tolerance and cytolytic responses by NK cells.
NK cell activation is regulated by a balance of signals derived from activating and inhibitory surface receptors. Activating receptors recognize an array of cell surface molecules, some of which are expressed ubiquitously on the surfaces of other cells of the body. Inhibitory receptors, such as KIR, recognize MHC class I (MHC-I) molecules, which are expressed on the surface of virtually every normal cell of the body, while many tumor cells lack expression of this “self” molecule. KIR engagement with MHC-I triggers inhibitory signals (predominantly protein tyrosine phosphatase-based) within the NK cell that dominantly suppress activating receptor signals (predominantly protein tyrosine kinase-based) and result in tolerance. Upon encounter with a rare tumor cell lacking MHC-I (so-called “missing self”), the lack of inhibitory signaling at the cell contact interface triggers rapid and directed release of cytolytic granules toward the tumor target cell, which results in specific cytolysis of the target cell.