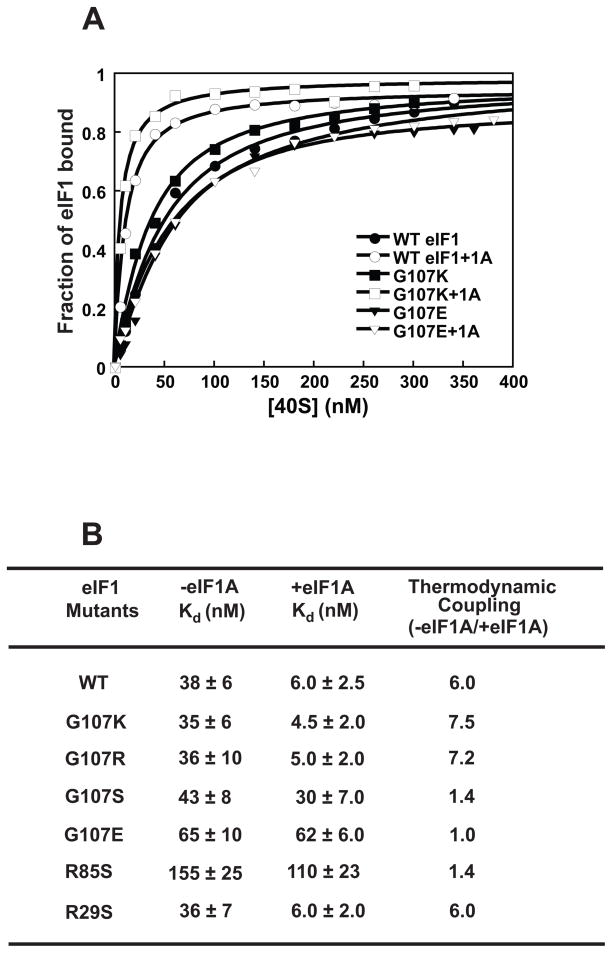
Figure 3. The charge at and around position 107 affects eIF1’s affinity for the 40S subunit and its thermodynamic coupling with eIF1A.
(A) Binding of fluorescein-labeled eIF1 mutants was monitored using fluorescence anisotropy: (●) WT eIF1; (○) WT eIF1 + eIF1A; (■) G107K; (□) G107K + eIF1A; (▼) G107E; (∇) G107E + eIF1A. Each curve is the average of at least three experiments. (B) Kd values for eIF1 mutants binding to the 40S subunit. The ratio of Kds in the absence and presence of eIF1A is the fold thermodynamic coupling between the two factors. Errors given are mean deviations.