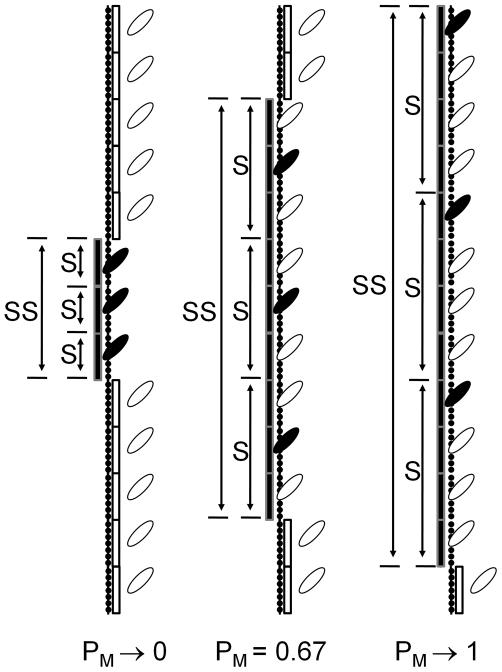
Figure 1. Novel myosin-based cooperative mechanism for vertebrate striated muscle.
The diagram depicts positions of tropomyosin (Tm) generated by interactions of troponin (Tn) and myosin with actin. An interaction between Tn and actin (not depicted) is energetically coupled to the stability of Tm in Position B (open rectangle), which blocks the association of myosin (open myosin head) with actin (filled monomers). An interaction of myosin with actin and Tm (closed myosin head) is coupled to a conformational change in Tm and the stability of Tm in Position M (closed rectangle). The conformational change stiffens one or more Tm subunits into a functional unit, referred to as a segment (S), which requires one coupled myosin and excludes all other myosin bound within S from being coupled. The stiffening of Tm requires multiple myosin to be coupled, but, since S can have only one coupled myosin, myosin from multiple S must cooperate to form a larger functional unit of Tm, referred to as a super segment (SS). Only one bound myosin per Tm subunit has the potential to be coupled within a segment, referred to as free myosin (open head attached to actin). Free myosin stabilizes the coupled state of myosin by being available to be coupled, as coupling within the segment is dynamic. The number of Tm subunits per S depends on the probability that myosin can be coupled (PM). The maximum number of Tm per S and the number of myosin that must be coupled to form SS are intrinsic properties of Tm arbitrarily chosen to be 4 and 3 respectively for this diagram.