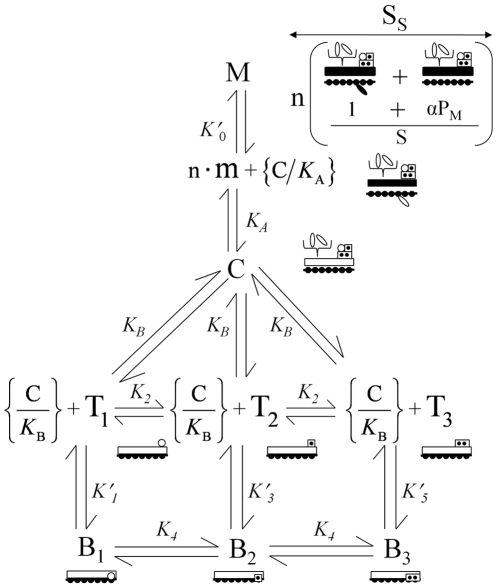
Figure 2. Annotated equilibrium model.
B1, B2, B3, C, and M represent mole fractions of binding states coupled to the positions of Tm denoted by the letters (blocking, central, and myosin dependent, respectively). B1, B2, and B3 are represented by Tm (open rectangle) held in a blocking position on actin by interactions between actin and Tn in each of three possible calcium bound states, namely, zero sites filled (open circle), one site filled (one dot), and two sites filled (two dots). Tn held by Tm in Positions C and M is uncoupled. T1, T2, and T3, represent the mole fractions of uncoupled Tn with zero, one, and two calcium bound respectively (single symbol with open circle, one dot, and two dots). T1, T2, and T3 must be carried by thermal motions of Tm to the vicinity of actin binding sites in Position B for coupling to occur. The mole fraction of Tm in Position B, {C/K B} (brackets denote non-equilibrium state), determines the mole fraction of actin binding sites available for interaction with T1, T2, and T3 (circle, one dot, and two dots dissociated from actin in Position B). The segment conformation of Tm (filled rectangle) requires the formation of a coupled myosin state (closed myosin head). The coupled myosin state is stabilized by the mole fraction of free myosin present in segments (open myosin head attached to actin) and the mole fraction of C transiently present in Position M (given by {C/K A}). Each segment is composed of a variable number of Tm subunits (1+αPM; PM is the probability of the M state) and a super segment is composed of n segments. The mole fractions of segments (S) and super segments (Ss) each equal M. Tm in Positions C and M supports cycling myosin intermediates (pair of myosin heads) for sliding filaments and isometric tension respectively. Omitted from the diagram for clarity are the redundant reactions for calcium binding to Tn in Positions C and M and the explicit reaction with actin that forms free myosin.