Table 3.
Yields and chirality transfer levels in cyclizations of enantiomeric precursors 15 to give 14a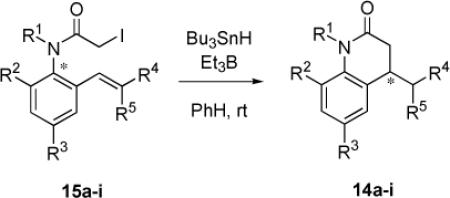
| entry | precursor | R1 | R2 | R3 | R4 | R5 | er | % yield 14b |
er | % cte |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (+)-15a | Me | Me | Me | CO2t-Bu | H | 98.5/1.5 | 79 | 91/9 | 92 |
| 2 | (−)-15a | Me | Me | Me | CO2t-Bu | H | 85/15 | 81 | 84/16 | 99 |
| 3 | (+)-15b | PMB | Me | Me | CO2t-Bu | H | 100/0 | 87 | 95/5 | 95 |
| 4 | (−)-15b | PMB | Me | Me | CO2t-Bu | H | 100/0 | 85 | 96/4 | 96 |
| 5 | (+)-15c | PMB | OMe | H | CO2t-Bu | H | 99/1 | 94 | 93/7 | 94 |
| 6 | (−)-15c | PMB | OMe | H | CO2t-Bu | H | 99/1 | 96 | 93/7 | 94 |
| 7 | (+)-15e | PMB | TMS | Me | CO2t-Bu | H | 100/0 | 95 | 95/5 | 95 |
| 8 | (−)-15e | PMB | TMS | Me | CO2t-Bu | H | 100/0 | 93 | 94/6 | 96 |
| 9 | (P)-15f | PMB | Br | Me | CO2t-Bu | H | 99/1 | 63 | 95/5 | 96 |
| 10 | (M)-15f | PMB | Br | Me | CO2t-Bu | H | 0/100 | 66 | 94/6 | 94 |
| 11 | (+)-15h | PMB | Me | Me | CN | H | 99.5/0.5 | 71 | 93/7 | 93e |
| 12 | (−)-15h | PMB | Me | Me | CN | H | 99/1 | 68 | 96/4 | 97e |
| 13 | (+)-15i | PMB | Me | Me | Me | Me | 99/1 | 57 | 80/20 | 81 |
| 14 | (−)-15i | PMB | Me | Me | Me | Me | 0/100 | 55 | 80/20 | 80 |
Conditions: Bu3SnH and Et3B in 20 mL PhH were added over 2 h via syringe pump to a stirred 10 mM PhH solution of 15.
Yield of 14 after isolation by column chromatography on 10% w/w KF/silica gel.
Absolute configuration determined by X-ray crystallography.
Percent chirality transfer.
Neat Bu3SnH and Et3B were added sequentially in one portion to a stirred PhH solution of iodide with [Bu3SnH]i = 5 mM.
