Abstract
Human adenovirus type 5 (Ad5) has been the most popular platform for the development of oncolytic Ads. Alternative Ad serotypes with low seroprevalence might allow for improved anticancer efficacy in Ad5-immune patients. We studied the safety and efficacy of rare serotypes Ad6, Ad11 and Ad35. In vitro cytotoxicity of the Ads correlated with expression of CAR and CD46 in most but not all cell lines. Among CAR-binding viruses, Ad5 was often more active than Ad6, among CD46-binding viruses Ad35 was generally more cytotoxic than Ad11 in cell culture studies. Ad5, Ad6, and Ad11 demonstrated similar anticancer activity in vivo, whereas Ad35 was not efficacious. Hepatotoxicity developed only in Ad5-injected mice. Predosing with Ad11 and Ad35 did not increase infection of hepatocytes with Ad5-based vector demonstrating different interaction of these Ads with Kupffer cells. Data obtained in this study suggest developing Ad6 and Ad11 as alternative Ads for anticancer treatment.
Keywords: adenovirus, oncolytic viruses, Kupffer cells, xenograft model antitumor assays, toxicity
Introduction
Replication-competent adenoviruses (Ads) are being developed as therapeutic agents for the treatment of cancer based on their lytic life cycle that allows for destruction of infected cells (Cattaneo et al., 2008). Human Ad type 5 (Ad5) is the most extensively used platform for the development of oncolytic Ads and their consequent testing in preclinical and clinical settings. Replication-competent Ad5-based vector H101 was recently approved for clinical use via local administration in patients with head-and-neck carcinoma in China (Garber, 2006). In most cases, oncolytic Ad5 is used by intratumoral injection. Systemic treatment with Ad5-based oncolytic viruses faces additional challenges due to the barriers to efficient virus delivery to the tumor site(s) (Demers et al., 2003). Administration of high doses of Ad5-based vectors results in the virus sequestration by the liver that limits the number of virions capable of reaching the tumor after systemic injection. The majority of the injected dose was shown to be taken up and destroyed by the liver macrophages (Kupffer cells) within the first 24 hours after the injection (Worgall et al., 1997). Additionally, infection of hepatocytes results in a dose-dependent hepatotoxicity (Shashkova et al., 2007) that limits the therapeutic window of the systemic treatment with Ad5-based oncolytic viruses (Small et al., 2006).
High global seroprevalence of Ad5 and high Ad5 neutralizing antibody (NAb) titers in human populations represent another significant concern for the systemic application of Ad5-based vectors. It is estimated that 37 to 90% of human subjects in Europe, the United States, Asia, and Africa have Ad5 NAb (Vogels et al., 2003; Holterman et al., 2004; Nwanegbo et al., 2004). Pre-existing NAb in patients is expected to inhibit the anticancer activity of intravascularly administered Ad (Chen et al., 2000). Therefore, it is of a particular interest to assess the possibility of using Ad serotypes alternative to Ad5 as oncolytic agents. There are more than 50 currently known Ad serotypes that are grouped into six subgroups (A through F) based on a number of properties including hemagglutination characteristics and DNA sequences. Replication-defective vectors based on Ad serotypes that have low seroprevalence and do not cross-react with Ad5 NAb were recently suggested as alternative vehicles for vaccination and gene therapy (Vogels et al., 2003; Holterman et al., 2004; Capone et al., 2006; Abbink et al., 2007; Seshidhar et al., 2003). However, anticancer activity of these serotypes has not been well characterized.
In this report, wild type (wt) Ad6, Ad11 and Ad35 were studied as the potential new oncolytic Ads in comparison with wt Ad5. Ad6, 11, and 35 were selected for their low seroprevalence and for their differing interactions with cellular receptors and blood factors. Ad5 and Ad6 viruses belong to group C and use Coxsackie-Adenovirus Receptor (CAR) as primary receptor for cell entry (Bergelson et al., 1997; Roelvink et al., 1998). Ad11 and Ad35 viruses, belonging to group B, use membrane cofactor protein CD46 as a cellular attachment protein (Segerman et al., 2003; Gaggar et al., 2003). Recently, binding of blood coagulation factor X to Ad hexon was identified as a major mechanism of Ad5 infection of hepatocytes in vivo (Waddington et al., 2008; Kalyuzhniy et al., 2008). Binding of factor X to Ad6 hexon by surface plasmon resonance was shown to occur with the same efficacy as to Ad5 (Waddington et al., 2008) whereas Ad11 and Ad35 lacked or had significantly reduced binding to factor X (Waddington et al., 2008; Kalyuzhniy et al., 2008). These data indicate that Ad5 and 6 should both infect liver efficiently by binding to blood factors whereas Ad11 and 35 may not. Variations in the virus-host and the virus-cell interactions determine the differences in Ad serotype-specific tropism, the ability to kill the infected cells, and in the efficiency of production and spread of the progeny virions. These factors have a potential effect on the oncolytic activity and the safety profile of the various Ad serotypes. In this study, we provide novel information on in vitro and in vivo characteristics of Ad6, Ad11, and Ad35 in comparison with that of Ad5 that allow the development of alternative Ad serotypes for the treatment of cancer.
Results
Receptor expression and anticancer activity of Ad serotypes in vitro
We studied the in vitro spread and anticancer activity of wild-type Ad5, Ad6, Ad11, and Ad35 against a range of cancers: human prostate carcinoma – LNCaP, DU145, PC3; human breast carcinoma – SKBr-3, MDA-MB-231, MDA-MB-468; human hepatocellular carcinoma – HepG2, Hep3B; and human ovarian carcinoma – SKOV-3, OVCAR-3. Given that Ad5 and 6 use CAR as a primary receptor and Ad11 and 35 use CD46 as a receptor, we compared the cell surface expression of these two receptors to the ability of the viruses to kill each of the cell lines. Cells were stained with unlabeled CAR or CD46 antibodies and then detected by flow cytometry after staining with phycoerythrin-labeled secondary antibody to approximate the relative levels of CAR and CD46 on the cells. Oncolytic activity was assessed by infecting each of the cell lines at various multiplicities of infection (MOIs) with each of the viruses and cell viability was measured by MTT assay 5 days after infection.
Prostate carcinomas
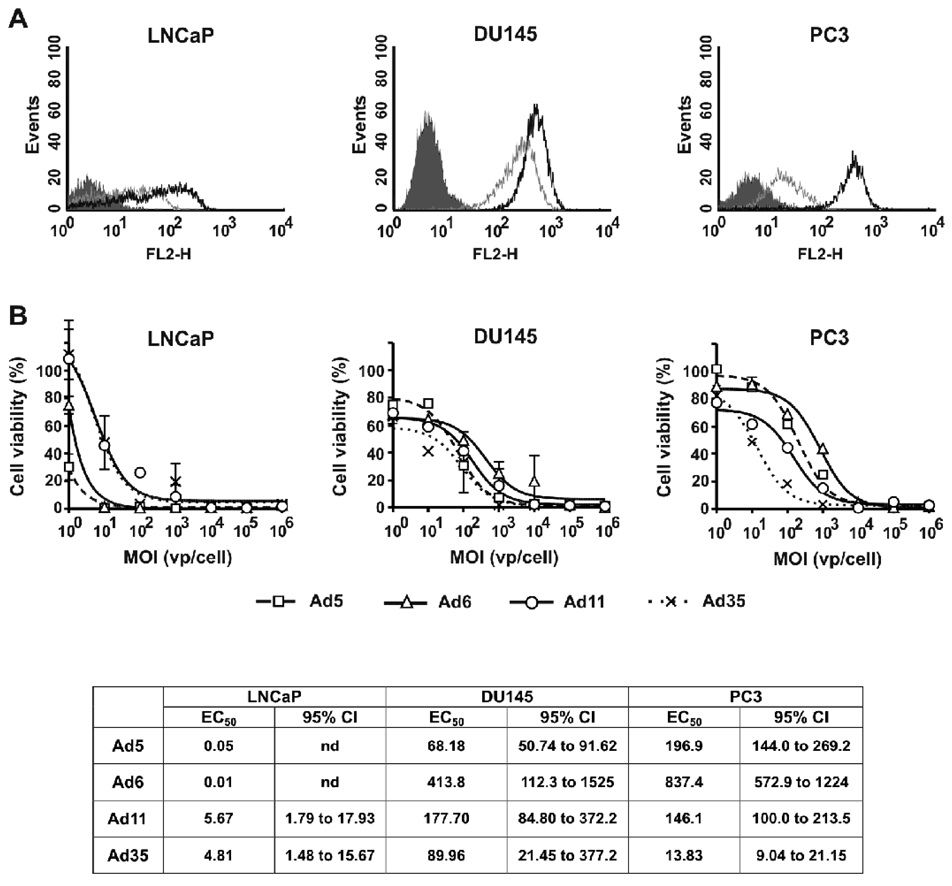
Each of the prostate cancer cell lines appeared to express higher levels of CD46 than CAR, but this did not generally translate into higher oncolytic activities by the CD46-binding viruses. LNCaP cells had 1.5-fold higher percentage of CD46-positive cells relative to CAR-expressing cells with 3-fold higher mean fluorescence intensity (MFI) of CD46 vs. CAR suggesting higher CD46 receptor density on the cells (Fig. 1A and Table 1). The differences between the dose-response curves were analyzed by ANOVA followed by Tukey’s HSD post-hoc test for pair-wise comparisons. Interestingly, CAR-binding Ad5 and Ad6 had significantly higher cytotoxicity at low MOIs relative to that of Ad11 and Ad35 (p<0.005 for Ad5 and Ad6 vs. Ad11 and Ad35) (Fig. 1B). There were no significant differences between the activities of the viruses belonging to the same group (Ad5 vs. Ad6 p=0.455, Ad11 vs. Ad35 p=0.999). PC3 cells had 2.7-fold higher percentage of CD46-expressing cells with 15-fold higher MFI as compared to CAR-positive cells (Fig. 1A and Table 1). In contrast to LNCaP cells, Ad infectivity profile of PC3 cells correlated with the receptor expression levels (Fig. 1B). CD46-binding Ad11 and Ad35 had significantly higher cytotoxicity at low MOIs in this cell line relative to that of Ad5 and Ad6 (p<0.001). There were no significant differences between dose-response curves for Ad5 and Ad6 (p=0.147), however, Ad35 was more cytotoxic as compared with Ad11 (P<0.001). The latter indicates the existence of intrinsic differences in Ad infectivity between the viruses from the same group. In a third prostate cell line, DU145, expression of both CAR and CD46 was detected on 100% of the cells with 2-fold higher CD46 cell surface density levels (Fig. 1A and Table 1). In this cell line, all viruses had similar dose-response cytotoxicity profiles (p>0.3) with exception of higher cytotoxicity of Ad35 relative to that of Ad6 (p=0.006) (Fig. 1B).
Fig. 1. Characterization of cell surface receptor expression profiles and cytotoxic activities of Ad serotypes in prostate cancer cell lines.
(A) FACS analysis of Ad receptors expressed by LNCaP, DU145, and PC3 cells: shaded line – Isotype control, grey line – CAR, black line – CD46. (B) Cells were infected with Ad serotypes at indicated MOIs (n=3). Cell viability was measured on day 5 after infection by MTT assay. Data presented as mean ± standard deviation (SD).
Table 1.
Cell surface expression profiles of Ad receptors.
| Cell origin | Cell line | CAR | CD46 | ||
|---|---|---|---|---|---|
| Positive cells (%) |
MFIa | Positive cells (%) |
MFIa | ||
| Prostate carcinoma | LNCaP | 49.61 | 8.69 | 72.96 | 27.43 |
| DU145 | 99.37 | 202.22 | 99.97 | 432.89 | |
| PC3 | 35.53 | 15.3 | 94.91 | 230.43 | |
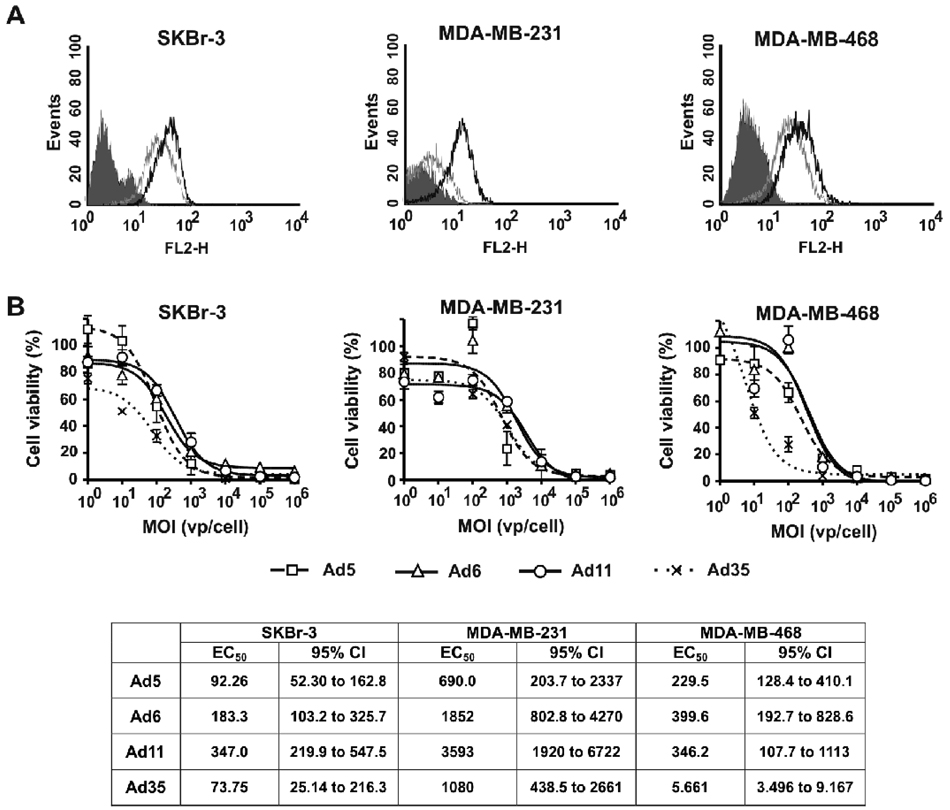
| Breast carcinoma | SKBr-3 | 97.77 | 23.14 | 99.91 | 34.5 |
| MDA-MB-231 | 7.91 | 10.35 | 75.85 | 40.69 | |
| MDA-MB-468 | 82.21 | 20.29 | 95.4 | 31.54 | |
|
Hepatocellular carcinoma |
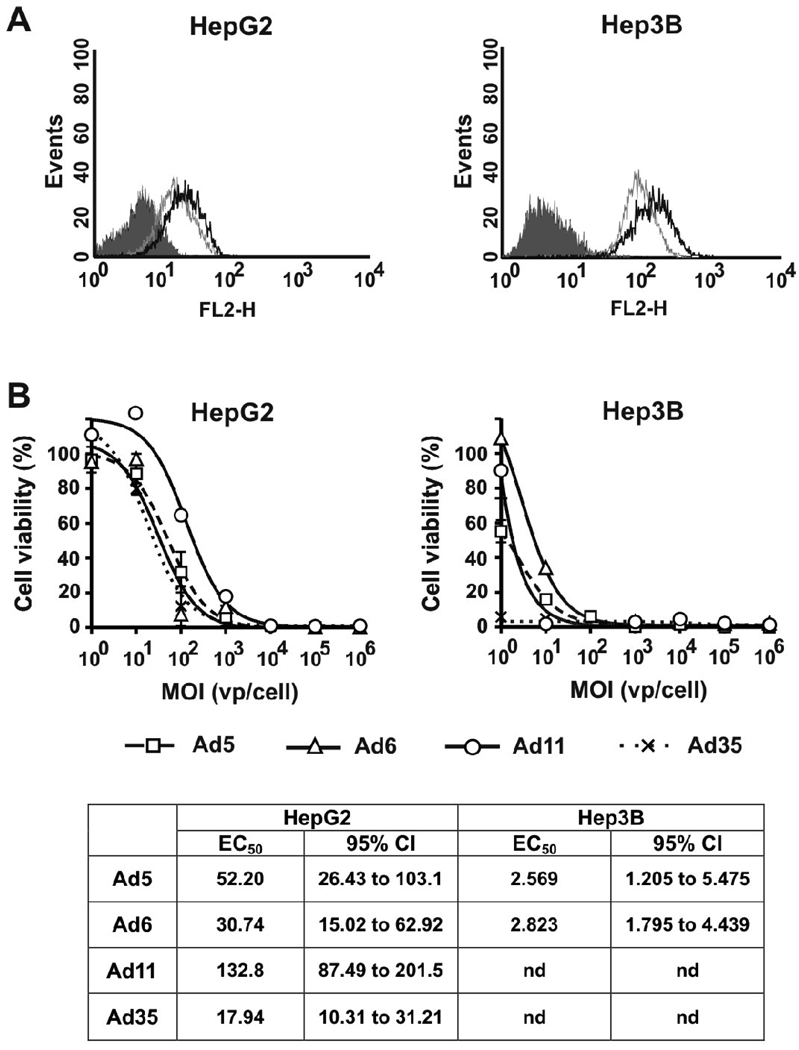
HepG2 | 67.15 | 15.49 | 82.62 | 20.47 |
| Hep3B | 98.02 | 90.43 | 99.15 | 141.23 | |
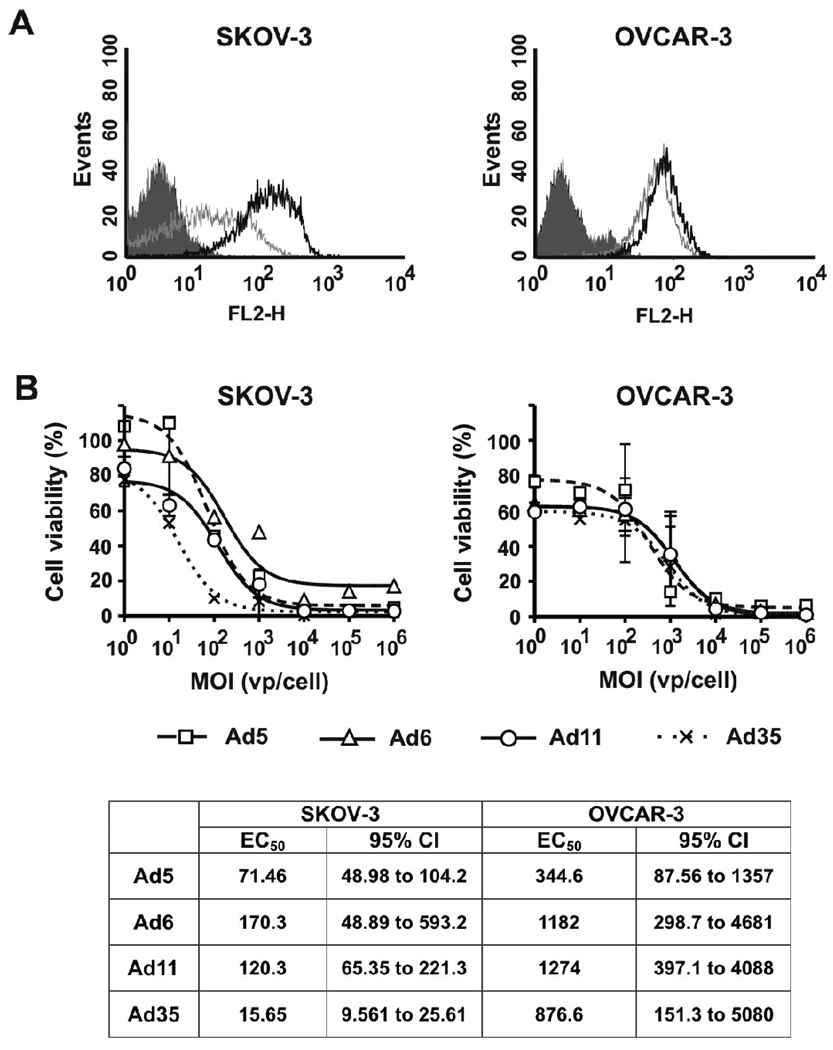
| Ovarian carcinoma | SKOV-3 | 65.94 | 12.43 | 99.07 | 104.29 |
| OVCAR-3 | 95.39 | 48.02 | 98.39 | 67.17 | |
Mean fluorescence intensity
Breast Carcinomas
Infection of breast cancer cell lines in general required higher MOIs for all of the Ads to reach EC50 (Fig. 2) as compared with that in prostate cancer cell lines (Fig. 1). This might be partially explained by the low cell surface CAR and CD46 receptor expression levels as determined by MFI levels on breast vs. prostate cancer cells (Fig. 2A and Table 1). SKBr-3 cells expressed slightly higher levels of CD46 as compared to CAR expression levels (Fig. 2A and Table 1). In this cell line, Ad35 was the most cytotoxic Ad relative to other serotypes (p<0.001), whereas Ad5, Ad6, and Ad11 were not significantly different from one another (p>0.980) (Fig. 2B). MDA-MB-231 cells had 9.6-fold higher percentage of CD46-expressing cells and 4-fold higher MFI relative to those of CAR-expressing cells (Fig. 2A and Table 1). The differences between the dose-response curves were analyzed by ANOVA followed by Tukey’s HSD post-hoc test for pair-wise comparisons. There were no significant differences in this cell line between the dose-response curves for Ads from the same group (p=0.758 for Ad5 vs. Ad6, p=0.935 for Ad11 vs. Ad35) (Fig. 2B). Ad6 had lowest cytotoxicity as compared with that of group B Ads (p=0.025 vs. Ad11, p=0.005 vs. Ad35), whereas Ad5 had intermediate activity that was not different from any other serotype (p=0.227 vs. Ad11, p=0.068 vs. Ad35). MDA-MB-468 cells expressed slightly higher levels of CD46 than CAR (Fig. 2A and Table 1). Ad35 had highest cytotoxic activity in this cell line relative to other Ad serotypes (p<0.002 vs. Ad5, Ad6, and Ad11) (Fig. 2B). In contrast, CD46-binding Ad11 had significantly lower activity relative to CAR-binding Ad5 (p=0.038) that was not different from the activity of CAR-binding Ad6 (p=1.000 for Ad11 vs. Ad6). These results demonstrate the lack of direct correlation between the expression of primary Ad receptors on the cell surface and the anticancer activity of Ad serotypes in MDA-MB-468 cells in vitro and suggest that other receptors or intracellular events play a distinct role in each Ad serotype infection process.
Fig. 2. Characterization of cell surface receptor expression profiles and cytotoxic activities of Ad serotypes in breast cancer cell lines.
(A) FACS analysis of Ad receptors expressed by SKBr-3, MDA-MB-231, and MDA-MB-468 cells: shaded line – Isotype control, grey line – CAR, black line – CD46. (B) Cells were infected with Ad serotypes at indicated MOIs (n=3). Cell viability was measured on day 5 after infection by MTT assay. Data presented as mean ± SD.
Hepatocellular carcinomas
HepG2 and Hep3B cell lines both expressed CAR and CD46. However the cell surface receptor levels were higher in Hep3B cells relative to those in HepG2 cells (Fig. 3A and Table 1). The differences between the dose-response curves were analyzed by ANOVA followed by Tukey’s HSD post-hoc test for pair-wise comparisons. There were no significant differences between dose-response curves for Ad5, Ad6, and Ad35 in HepG2 cells (p<0.900) (Fig. 3B). Ad11 had significantly lower cytotoxicity in this cell line relative to that of all other serotypes (p<0.001). In Hep3B cells, Ad6 had lowest cytotoxicity (p<0.010 vs. Ad5, Ad11, and Ad35) while Ad35 had the highest activity (p≤0.001 vs. Ad5, Ad6, and Ad11) (Fig. 3B). There was no significant difference between the dose-response curves for Ad5 and Ad11 (p=0.475).
Fig. 3. Characterization of cell surface receptor expression profiles and cytotoxic activities of Ad serotypes in hepatocellular carcinoma cell lines.
(A) FACS analysis of Ad receptors expressed by HepG2 and Hep3B cells: shaded line – Isotype control, grey line – CAR, black line – CD46. (B) Cells were infected with Ad serotypes at indicated MOIs (n=3). Cell viability was measured on day 5 after infection by MTT assay. Data presented as mean ± SD.
Ovarian carcinomas
In ovarian carcinoma cell line SKOV-3, staining for CD46 demonstrated 8.4-fold higher MFI than for CAR (Fig. 4A and Table 1). The differences between the dose-response curves were analyzed by ANOVA followed by Tukey’s HSD post-hoc test for pair-wise comparisons. The receptor expression profile correlated with higher cytotoxicity of CD46-binding Ads relative to that of CAR-binding viruses (p<0.001 for Ad11 and Ad35 vs. Ad5 and Ad6) (Fig. 4B). There was no significant difference between dose-response curves for Ad5 and Ad6 (p=0.286), whereas Ad35 was more cytotoxic at low MOIs than Ad11 (p=0.016). Another ovarian carcinoma cell line, OVCAR-3, expressed similar levels of CD46 and CAR (Fig. 4A and Table 1) and comparison of the dose-response curves did not detect significant differences between any of the Ad serotypes in this cell line (p>0.551) (Fig. 4B).
Fig. 4. Characterization of cell surface receptor expression profiles and cytotoxic activities of Ad serotypes in ovarian carcinoma cell lines.
(A) FACS analysis of Ad receptors expressed by SKOV-3 and OVCAR-3 cells: shaded line – Isotype control, grey line – CAR, black line – CD46. (B) Cells were infected with Ad serotypes at indicated MOIs (n=3). Cell viability was measured on day 5 after infection by MTT assay. Data presented as mean ± SD.
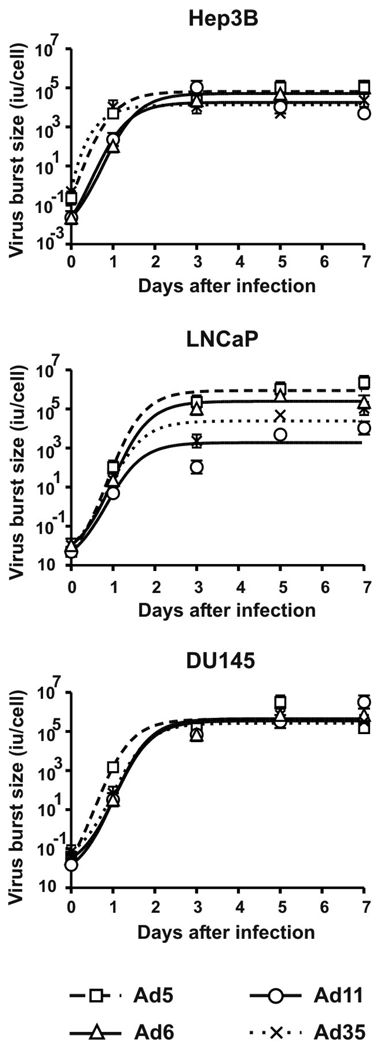
One-step growth curve
To assess amplification ability of Ad serotypes on different cancer cells, we infected Hep3B, LNCaP, and DU145 cells with 100 virus particles (vp) of Ad5, Ad6, Ad11, and Ad35 for 2 hours and determined total virus yields in infectious units (iu) on days 0, 1, 3, 5, and 7 after the infection (Fig. 5). Under these conditions, all cells are synchronously infected. All of the viruses produced 103 to 105 infectious progeny viruses from each cell. When analyzed with Repeated Measures ANOVA, no significant differences were found in the kinetics of the burst size of any of the Ads in Hep3B (p=0.634) and DU145 (p=0.638) cells. In contrast, virus burst sizes varied significantly in LNCaP cells (p=0.050). Group B Ad11 and Ad35 produced lower yields relative to group C Ad5 and Ad6 yields in LNCaP cells. These results could explain lower cytotoxicity of Ad11 and Ad35 in this cell line (Fig. 1B). These data are in agreement with recently reported lower replicative capacity of Ad11-based vector in LNCaP cells as compared with DU145 cells (Sandberg et al., 2009). Cell surface molecule profiling suggested that DU145 cells represent more progressed cancer cell type than LNCaP (Liu, 2000) suggesting perhaps that stage-dependent variations in the intracellular pathways might explain the inefficient replication of group B Ads in the LNCaP cells.
Fig. 5. One-step growth curves of Ad serotypes.
Hep3B, LNCaP, and DU145 cells were infected with Ad5, Ad6, Ad11, or Ad35 at MOI of 100 vp/cell for 2 hrs. After washing three times, fresh medium was added to the cells (day 0 timepoint). Cells and supernatants were collected at indicated timepoints and total infectious virus yield was determined by limiting dilution assay on A549 cells.
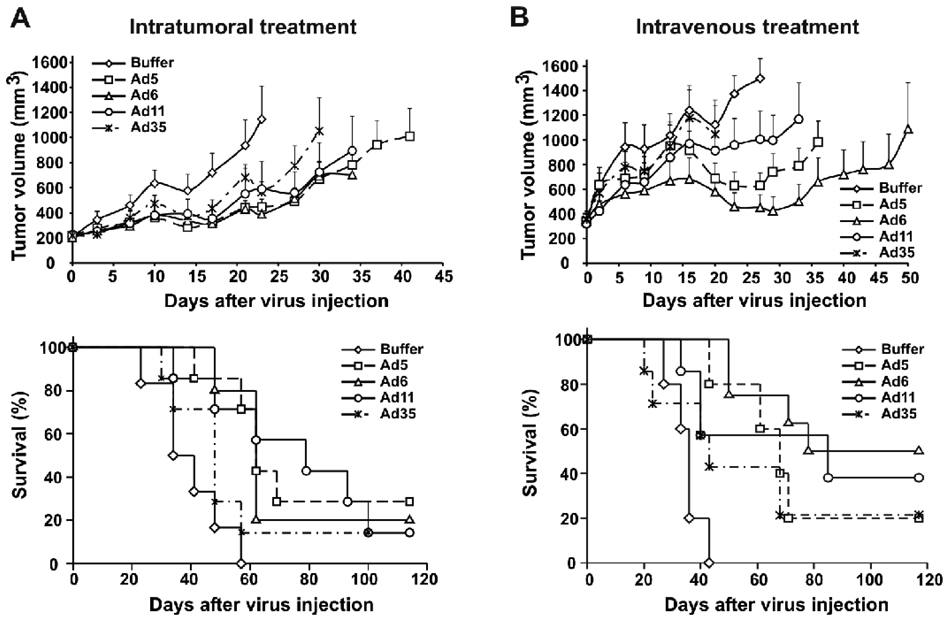
Oncolytic activity of Ad serotypes in prostate tumor model in vivo
To assess the oncolytic potential of alternative Ad serotypes in vivo, we tested the viruses by intratumoral and intravenous injection in established subcutaneous DU145 tumors in nude mice. DU145 were selected as all of the viruses generated similar burst sizes (Fig. 5), and had similar EC50 values, although they varied from 68 to 413 (Fig. 1). Based on in vitro EC50 data, one would predict efficacy vs DU145 of Ad5 > Ad35 > Ad11 > Ad6. Based on in vitro burst size data, one would predict similar efficacies by the viruses.
We first treated mice bearing DU145 subcutaneous tumors by a single intratumoral injection of 3×1010 vp of Ad5, Ad6, Ad11 or Ad35. Tumor growth was inhibited in all virus-treated groups (Fig. 6A top) with strongest effects produced by Ad5 and Ad6 (p=0.034 and p=0.025, respectively, vs. Buffer on day 23 after start of treatment). Ad11 and Ad35 tumor suppressing activity did not reach statistical significance on day 23 (p=0.131 and p=0.104, respectively, vs. Buffer) but it was not different from that of Ad5 and Ad6 (p>0.9). Interestingly, when longer term effects of the treatments were assessed by survival analysis, Ad5, Ad6, and Ad11 were found to significantly prolong the survival of the treated mice (p=0.003, p=0.004 and p=0.008, respectively, vs. Buffer) whereas Ad35 did not increase survival times (p=0.26 vs. Buffer) (Fig. 6A bottom). The median survival time for buffer-treated mice was 34 days, for Ad35 was 48 days, for Ad5 was 62 days, for Ad6 was 62 days, and for Ad11 was 79 days. The increased survival times produced by Ad5, Ad6, or Ad11 treatment were not statistically different from each other (p>0.836).
Fig. 6. Anticancer activity of Ads in DU145 tumor model.
(A) Subcutaneous DU145 tumors (average tumor volume 212 mm3) were injected once intratumorally with 100 µl of buffer or 3×1010 vp of Ad5, Ad6, Ad11, or Ad35 (n=6 or 7) in 100 µl volume on day 0. (top) Tumor volumes were measured twice a week. Average tumor volumes are shown up to the last day when all animals were alive in a group. Mice were euthanized when tumor volume reached 2000 mm3. Data presented as mean + standard error (SE). (bottom) Kaplan-Meyer survival curves for mice from (top) were plotted. (B) Mice bearing subcutaneously established DU145 tumors (average tumor volume 345 mm3) received two intravenous injections of 100 µl buffer or 3×1010 vp of Ad5, Ad6, Ad11, or Ad35 (n=7 or 8) in 100 µl volume on day 0 with 4 hours separating the injections. (top) Measurements of tumor volumes were taken twice a week. Average tumor volumes are shown up to the last day when all animals were alive in a group. Mice were euthanized when tumor volume reached 2000 mm3. Data presented as mean + SE. (bottom) Kaplan-Meyer survival curves for mice from (top) were plotted.
The oncolytic activity of the viruses was next tested for systemic administration after intravenous (i.v.) injection. It is known that Ad5-based vectors are rapidly cleared from the bloodstream by liver macrophages after i.v. injection (Worgall et al., 1997) leading to reduced efficacy of systemic anticancer treatment (Shashkova et al., 2008a). Uptake of systemically delivered Ad5 by Kupffer cells results in death of these cells that prevents further uptake of the second dose of the virus (Manickan et al., 2006). We applied a two-dose regimen of systemic treatment of DU145 tumors with the Ad serotypes. A first “predose” was injected i.v. to deplete Kupffer cells and then 4 hours later, a second i.v. dose of the virus was given as in (Shashkova et al., 2008a). Analysis of the tumor volumes in Ad5, Ad6, and Ad11-treated groups relative to those in buffer-treated group was performed on day 27 after the start of treatment that was the last day when all of animals in the buffer-treated group still had tumors of less than 2000 mm3 volume (Fig. 6B top). Systemic treatment with Ad5 and Ad6 produced significant inhibition of tumor growth (p=0.010 and p=0.001, respectively, vs. Buffer) with similar levels of tumor suppression (p=0.851 for Ad5 vs. Ad6). Ad11 had intermediate activity after systemic injection (p=0.200 vs. Buffer; p=0.376 vs. Ad5; p=0.071 vs. Ad6). Analysis of tumor volumes in Ad35-treated group on day 20 demonstrated lack of significant anticancer activity (p=0.999 vs. Buffer).
The median survival time for buffer-treated mice was 36 days, for Ad35 was 43 days, for Ad5 was 68 days, for Ad6 was 78 days, and for Ad11 was 85 days (Fig. 6B bottom). Systemic treatment with Ad5, Ad6, and Ad11 significantly prolonged survival of the mice (p=0.003, p<0.001, and p=0.023 for Ad5, Ad6, and Ad11, respectively, vs. Buffer). Ad35 had intermediate activity (p=0.160 vs. Buffer; p=0.476 vs. Ad5, p=0.085 vs. Ad6, p=0.435 vs. Ad11). There were no significant differences between survival times in Ad5, Ad6, and Ad11 treated groups (p>0.215).
In summary, in vitro data would have predicted equal oncolytic activities by the viruses or rank efficacies of Ad5 > Ad35 > Ad11 > Ad6. Actual in vivo data demonstrated the rank of efficacies of Ad5 = Ad6 = Ad11 > Ad35.
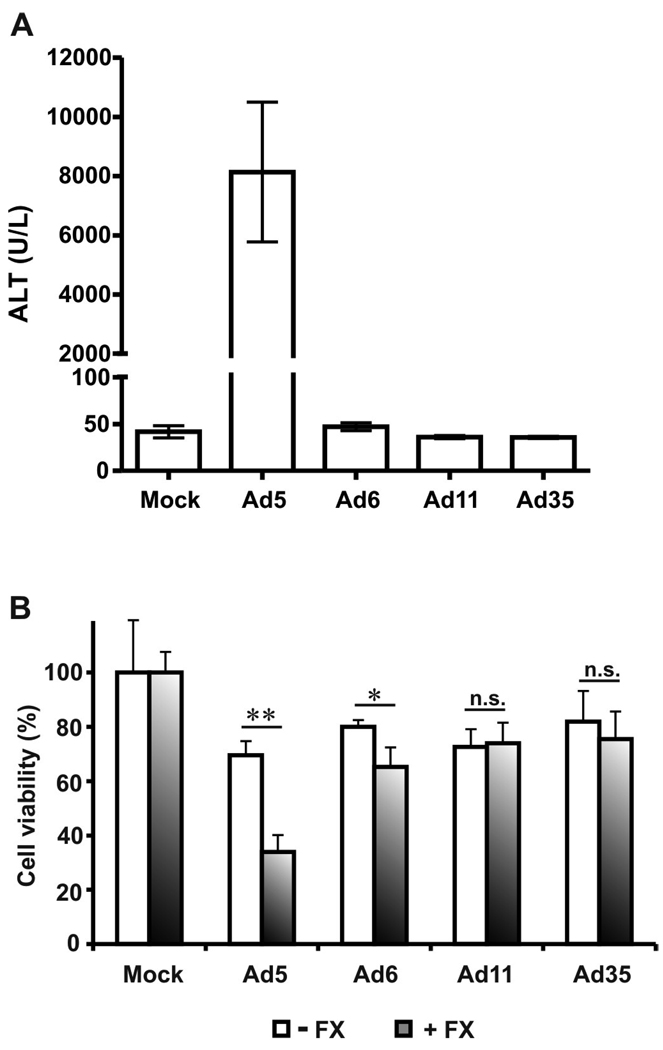
Lack of liver toxicity of Ad6, Ad11, and Ad35 after high-dose injection into CD46 transgenic mice
The viruses used in this study are fully replication-competent wt viruses. They would be expected to have off-target toxicities prior to being modified for cancer-specificity by transcriptional or transductional targeting. To determine hepatotoxicity of Ad serotypes, we injected CD46 transgenic mice intravenously with high dose (1.5×1011 vp) of Ad5, Ad6, Ad11, and Ad35 and measured serum levels of liver enzyme alanine aminotransferase (ALT) on day 3 after injection (Fig. 7A). CD46 transgenic mice were shown to have human-like tissue specificity of CD46 expression (Mrkic et al., 1998) and were used to provide Ad11 and Ad35 their cognate receptor that is lacking in wild-type mice.
Fig. 7. Hepatotoxicity of Ad serotypes and effect of factor X on Ad cytotoxicity.
(A) CD46-transgenic C57BL/6 mice were injected intravenously with 100 µl of buffer or 1.5×1011 vp of Ad5, Ad6, Ad11, or Ad35 (n=3) in 100 µl volume on day 0. Blood was collected on day 3 after injection and serum ALT levels were measured. (B) Ads were preincubated with buffer or factor X for 10 min. at 37°C and then added to HepG2 cells at 1000 vp/cell. Two hours later the cells were washed and the medium was replaced. Cell viability was measured on day 3 after infection. Data presented as mean + SD. * - p=0.008, ** - p<0.001, n.s. – not significant.
Mice injected intravenously with Ad5 became moribund and were sacrificed on day 3. The morbidity correlated with extremely high ALT levels in these mice (p<0.001 vs. Mock, Ad6, Ad11, and Ad35). In contrast, Ad6, Ad11, and Ad35 injections did not increase ALT levels (p=1.000 vs. Mock) demonstrating lack of liver damage induced by these Ad serotypes.
Binding of hexon to blood factor X was shown to be crucial for Ad5 infection of hepatocytes (Waddington et al., 2008; Kalyuzhniy et al., 2008; Vigant et al., 2008). We have shown previously that intervention into this pathway by anticoagulant treatment reduces hepatotoxicity of oncolytic Ad5-based vector (Shashkova et al., 2008a). The lack of liver toxicity of Ad11 and Ad35 correlates with the finding that these Ad serotypes have weak or absent binding to factor X (Waddington et al., 2008; Kalyuzhniy et al., 2008). However, Ad5 and Ad6 were shown to bind factor X with equal efficiency by surface plasmon resonance (Waddington et al., 2008). The difference in hepatotoxicity profiles of Ad5 and Ad6 (Fig. 7A), therefore, was unexpected. To evaluate the potential mechanism of reduced liver toxicity of Ad6, we infected HepG2 cells with Ad serotypes in the presence or absence of factor X and measured cell viability on day 3 after infection. We found that factor X significantly increased cytotoxicity of Ad5 (p<0.001) and Ad6 (p=0.008), but not Ad11 (p=0.799) and Ad35 (p=0.431) (Fig. 7B). These results correlate with Ad serotype-specific capability to bind factor X. Interestingly, presence of factor X mediated significantly stronger effect in Ad5-infected group relative to that in Ad6-infected group (decrease in cell viability 36% vs. 15% for Ad5 and Ad6, respectively, p=0.002). As these Ad serotypes bind factor X with equal efficiency, the differences in factor X-mediated potentiation of cytotoxicity suggest the existence of additional mechanisms influencing the infection.
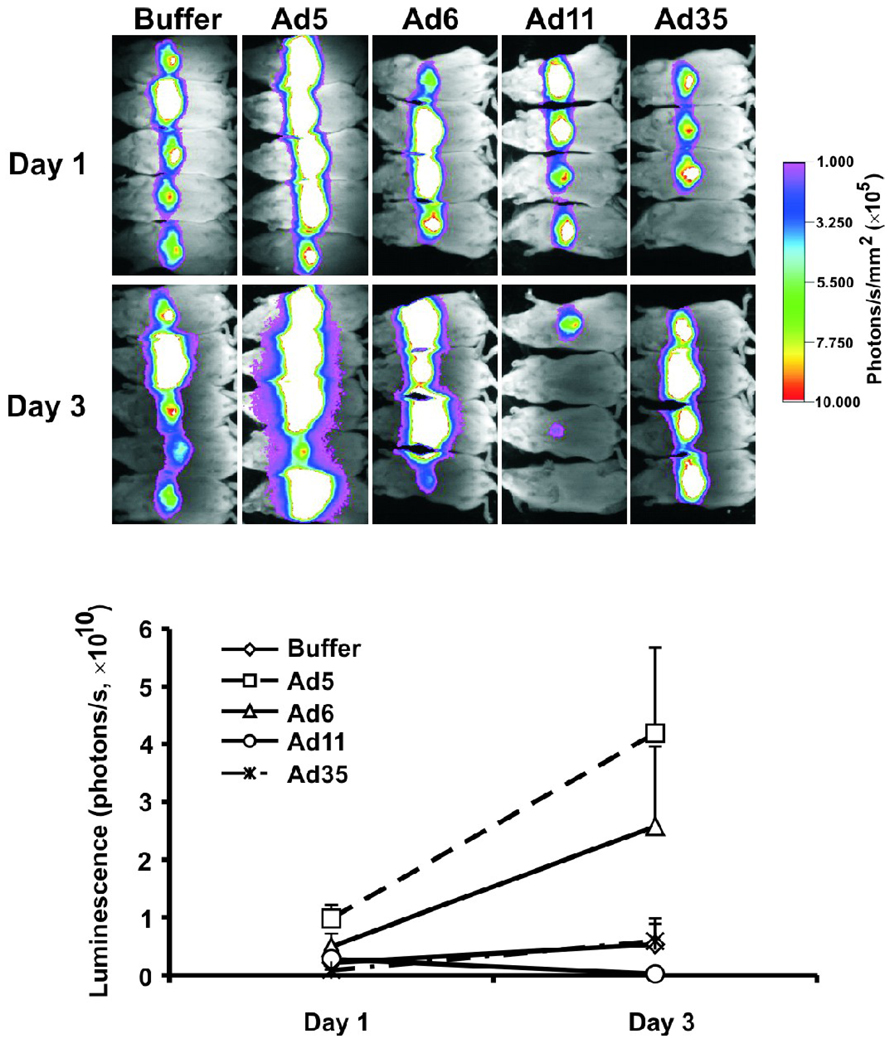
Effects of Ad serotypes on Kupffer cells after i.v. injection
We have previously shown that clearance of i.v. injected oncolytic Ad5-based vector by Kupffer cells reduced activity of systemic anticancer therapy (Shashkova et al., 2008a). It is important, therefore, to determine whether alternative Ad serotypes also interact with liver macrophages. Intravenous injection of Ad5-based vectors results in rapid necrosis of Kupffer cells in mice (Manickan et al., 2006; Xu et al., 2008). Injection of second dose of Ad5 vector leads to increased infection of hepatocytes that can be detected by higher transgene expression (Tao et al., 2001; Shashkova et al., 2008a). To assess whether Ad6, Ad11, and Ad35 have similar predosing effects on Kupffer cells, we pre-injected mice with buffer or 3×1010 vp of each Ad serotype and 4 hours later injected mice with 3×1010 vp of Ad5-based vector expressing reporter gene for firefly luciferase. Mice were imaged for luciferase expression in the liver on days 1 and 3 after the injections (Fig. 8) and quantified luminescence levels were analyzed with Repeated Measures ANOVA followed by Tukey’s HSD test. As expected, predosing with Ad5 increased luciferase expression from the Ad5 vector in the liver (p=0.019 vs. Buffer). Interestingly, predosing with Ad11 and Ad35 did not result in significant changes in the luminescence (p=0.997 and p=1.000 for Ad11 and Ad35, respectively, vs. Buffer). The predosing effect of Ad5 was significantly different from that of Ad11 (p=0.014) and Ad35 (p=0.025). In contrast, predosing with Ad6 produced intermediate results with luminescence levels in the liver not different from the levels in either group (p=0.562 vs. Ad5, p=0.433 vs. Buffer, p=0.320 vs. Ad11, p=0.456 vs. Ad35).
Fig. 8. Effect of Ad predosing on Kupffer cells.
ICR mice were predosed intravenously with buffer or 3×1010 vp of Ad5, Ad6, Ad11, or Ad35 (n=4 or 5) followed 4 hours later with intravenous injection of buffer or 3×1010 vp of Ad5-based vector expressing firefly luciferase reporter gene. Luciferase expression was imaged on days 1 and 3 after injection and was quantified. Data presented as mean + SE.
Discussion
To date, Ad5 has been the most studied Ad serotype used as a backbone for the development of vectors for vaccinations, anticancer treatment, and gene therapy. This is partially explained by the knowledge obtained during the last two decades about Ad5 (Campos & Barry, 2007; Rux & Burnett, 2004). While recombinant DNA technologies made possible significantly changing natural characteristics of Ad5 in order to improve its therapeutic activity (Doronin et al., 2000; Barton et al., 2006; Post et al., 2007; Shashkova et al., 2008b), other Ad serotypes have been generally neglected as potentially valuable agents for the treatment of cancer. The existence of more than 50 Ad serotypes with differences in receptor interactions, blood factor interactions, safety, and seroprevalence suggests the possibility of increasing the portfolio of therapeutic viruses by choosing those with most attractive features. In this report we describe in vitro and in vivo characteristics of wt Ad serotypes 6, 11, and 35 with regard to the development of these viruses as alternative or additional to Ad5 oncolytic agents. Low global seroprevalence and relatively well known biological characteristics of these Ads suggest their potential therapeutic value (Vogels et al., 2003; Holterman et al., 2004; Abbink et al., 2007; Capone et al., 2006; Seshidhar et al., 2003).
Group C Ad5 and Ad6 viruses and group B Ad11 and Ad35 viruses use CAR and CD46, respectively, as primary receptors for cell entry (Bergelson et al., 1997; Roelvink et al., 1998; Segerman et al., 2003; Gaggar et al., 2003). CD46 is ubiquitously expressed on human cells, whereas CAR expression varies depending on the cell type. It was recently reported that in addition to CD46, Ad11 uses unidentified receptor X that is expressed at high levels on human tumor cells, whereas Ad35 nearly exclusively uses CD46 for cell infection (Tuve et al., 2006). Heparan sulfate proteoglycans (HSPG) were shown to play a role in infection with Ad5 and Ad35 (Dechecchi et al., 2000; Tuve et al., 2008). Group C Ads use αvβ1,3,5 integrins as secondary receptors for virus internalization (Wickham et al., 1993; Li et al., 2001). Additionally, intracellular trafficking pathways differ for CAR- and CD46-binding viruses (Leopold & Crystal, 2007). It was demonstrated that group B viruses accumulate in lysosomes whereas group C viruses rapidly traffic to the nucleus.
We found that infection of human cancer cell lines of prostate, breast, ovarian, and hepatocellular origin with Ad5, 6, 11, and 35 resulted in different levels of cytotoxicity. In vitro, Ad5 was generally more active than Ad6, and Ad35 had higher cytotoxicity as compared with that of Ad11. The activity of Ads correlated with CAR and CD46 expression levels in most but not all cell lines. The cytotoxicity of the viruses did not correlate with the receptor expression profile in LNCaP cells. Despite lower level of CAR expression relative to that of CD46, CAR-binding Ad5 and Ad6 had significantly higher activity in LNCaP cells than Ad11 and Ad35. In addition, group C Ads had up to 100-fold higher burst size in LNCaP cells relative to that of group B Ads. This data are in agreement with recently reported lower cytotoxic activity of Ad11-based vector in LNCaP cells in comparison with other prostate cancer cell lines (Sandberg et al., 2009) that might be explained by the earlier cancer stage origin of this cell line (Liu, 2000). In contrast, we have not observed significant differences in the yields of Ad serotypes produced by infected Hep3B and DU145 cells. Variations in receptor(s) used for cell entry and intracellular trafficking specific for a particular Ad serotype most likely account for the observed differences in cytotoxicity and growth characteristics of the viruses.
Oncolytic activity of Ad serotypes in vivo was assessed in a prostate tumor xenograft model. Based on in vitro EC50 data, we would have predicted efficacy rankings of Ad5 > Ad35 > Ad11 > Ad6. Based on in vitro burst size data, we would have predicted similar efficacies by all of the viruses. After single, intratumoral injections, Ad5, Ad6, and Ad11 significantly prolonged survival of the mice. Surprisingly, treatment with Ad35 did not have significant impact on the survival rates. Similar results were obtained after systemic antitumor treatment with the Ad serotypes. Therefore, in vitro efficacies did not absolutely predict in vivo activities.
The results with Ad35 were unexpected as this virus demonstrated high anticancer activity in vitro. As nude mice do not ubiquitously express CD46 and DU145 tumor cells express high levels of CD46, theoretically, it is expected that these tumors cells should be readily infected with Ad35 in vivo. Systemic injection of Ad35-based vector into C57BL/6 mice led to extremely low levels of the virus genome in the liver, lungs, spleen, and bone marrow (Seshidhar et al., 2003) that was thought to be mediated by the lack of CD46 expression. However, low transduction efficiency of Ad35 vectors was also observed in CD46 transgenic mice (Sakurai et al., 2006) and in nonhuman primates that ubiquitously express CD46 (Sakurai et al., 2008). Inaccessibility of CD46 for infection due to predominant expression on the basolateral side of the cells was suggested as a possible mechanism for the discrepancy between in vitro and in vivo transductional characteristics of Ad35 (Sakurai et al., 2008). While this barrier could mediate lower anticancer activity of Ad35 applied systemically due to reduced binding of the virus to polarized endothelial cells, it appears that this mechanism would have small impact on the transduction of tumor cells after intratumoral virus delivery. Inefficient anticancer activity of Ad35 after intratumoral injection, therefore, indicates that other mechanisms are involved in Ad35 activity in vivo. Future studies are needed to elucidate the details of Ad35-host interactions to reveal potential strategies to translate high in vitro anticancer activity of this virus to the corresponding in vivo efficacy.
In contrast to Ad35, CD46-utilizing Ad11 had significant oncolytic activity after intratumoral and intravenous injections. These results suggest fundamental differences in the biologies of Ad11 and Ad35, perhaps related to alternate receptor utilization (e.g. receptor X), different intracellular events, or as yet unknown differences in pharmacological characteristics.
Ad6 and Ad11 in our experiments had shown anticancer activity similar to Ad5 after both intratumoral and intravenous injections suggesting that these Ad serotypes could be successfully used as additional or alternative to Ad5 oncolytic Ads. To the best of our knowledge, this is the first report on in vivo oncolytic characteristics of these Ads in comparison with that of Ad5. The feasibility of further increasing potency of Ad11 was recently demonstrated by applying a “directed evolution” approach (Kuhn et al., 2008) and creating a complex Ad3/Ad11 chimeric virus that had higher efficacy and safety as compared with the parental viruses.
Ad5 efficiently infects hepatocytes in vivo via binding of blood factor X to a capsid protein hexon (Waddington et al., 2008; Kalyuzhniy et al., 2008; Vigant et al., 2008). Ad6 hexon was shown to bind factor X with same efficiency as does Ad5 hexon (Waddington et al., 2008). In contrast, group B Ads do not bind factor X efficiently (Waddington et al., 2008; Kalyuzhniy et al., 2008). From this, we predicted that subgroup C Ad5 and 6 would cause hepatotoxicity whereas subgroup B Ad11 and 35 would not. We found that high dose i.v. injection of Ad serotypes in CD46 transgenic C57BL/6 mice produced high serum ALT levels and consequent morbidity only in Ad5-injected mice. In contrast, mice injected with the same dose of Ad6, Ad11, or Ad35 did not develop hepatotoxicity. Ad11 and Ad35 genomes were previously shown to accumulate in the liver of CD46-transgenic mice to levels similar to Ad5 (Stone et al., 2007). Absence of liver toxicity of group B Ads is in agreement with the fact that these viruses do not bind blood factor X. However, striking difference between the levels of liver damage produced by Ad5 and Ad6 cannot be explained by the differences in factor X binding. Addition of factor X increased cytotoxicity of both Ad5 and Ad6 in HepG2 cells in vitro and did not have this effect on Ad11 and A35 activity. These data confirmed that Ad serotype specific binding to factor X has functional effect on replicative virus infection. However, increase in cytotoxicity mediated by the presence of factor X was higher for Ad5 relative to Ad6. It is possible that length difference between fiber shafts mediates the outcome of Ad5 and Ad6 infections (Adhikary et al., 2004; Shayakhmetov & Lieber, 2000). Ad5 fiber has 21 β-turn repeats whereas Ad6 has only 18 (Adhikary et al., 2004); this difference may affect infection of hepatocytes. Notably, the shaft length difference does not negatively affect cancer cell infection in vitro or in vivo.
The difference in infectivity by Ad5 and Ad6 is consistent with observations in humans. Ad5 is responsible for 18.6% of all Ad infections in humans, compared with only 4% caused by Ad6 (Schmitz et al., 1983). Ad6 is uncommon in the adenoids and tonsils compared to other group C Ads (Garnett et al., 2009). In addition, Ad6 is the only member of group C Ads that has not been observed in immunosuppressed patients receiving liver transplants (Kojaoghlanian et al., 2003). Together with high anticancer activity after local and systemic administrations, these data suggest the development of Ad6 as a novel Ad serotype for oncolytic treatment.
Systemic treatment with oncolytic Ad5-based vectors is challenging due to the sequestration of the majority of the injected virus dose by liver Kupffer cells (Worgall et al., 1997) resulting in a rapid death of these cells (Manickan et al., 2006). Depletion of Kupffer cells with Ad5 pre-administration was shown to increase the levels of hepatocyte transduction with subsequently delivered Ad5-based vectors (Tao et al., 2001; Shashkova et al., 2008a). It was demonstrated that scavenger receptors on Kupffer cells are predominantly responsible for clearance of Ad5 vectors (Xu et al., 2008). Opsonization with natural antibody and complement was shown to play a contributory role to this process (Xu et al., 2008). It is not known whether other Ad serotypes have similar effect on Kupffer cells. As Kupffer cell mediated clearance of i.v. injected Ad5 reduces its therapeutic activity, it is important to determine whether alternative Ad serotypes also interact with these cells.
We show here that predosing mice with Ad5 and Ad6 increases transduction of hepatocytes with consequently injected Ad5 vector expressing firefly luciferase. Ad5 produced slightly higher increase in luciferase expression relative to that of Ad6. In contrast, predosing with Ad11 and Ad35 did not increase transgene expression in the liver. These data suggest that group C Ads interact with Kupffer cells in a similar manner whereas group B Ads either are not recognized by Kupffer cells or do not cause death of these cells. It was reported that killing of Kupffer cells by Ad5 occurred at post-binding step and was dependent on the intracellular trafficking of the vector, in particular, on the ability of the vector to escape endosomes (Smith et al., 2008). As accumulation of recombinant Ad5 vectors by Kupffer cells did not differ regardless of their levels of toxicity for Kupffer cells (Smith et al., 2008), it is possible that group B Ads bind these cells but do not cause their death due to the slower release from the endosomes (Leopold & Crystal, 2007). Fiber shaft was also shown to play significant role in depleting Kupffer cells as Ad5 with shorter Ad35 fiber was less effective at killing Kupffer cells (Smith et al., 2008). Shorter Ad6 fiber shaft relative to Ad5 fiber shaft (Adhikary et al., 2004) may represent a possible mechanism for the difference in predosing effects of Ad5 and Ad6. Further studies are warranted to investigate detailed interactions of various Ad serotypes with Kupffer cells and other types of cells in the liver.
In conclusion, we have shown that Ad5, Ad6, Ad11, and Ad35 produce cytotoxicity in various cancer cell lines in vitro. Ad5, Ad6, and Ad11 had significant anticancer activity after intratumoral and intravenous administration in mice bearing prostate tumor xenografts. Oncolytic activity of Ad35 was not significant in this tumor model despite the high cytotoxicity observed in vitro. Ad group specific differences in interaction with Kupffer cells provide additional information about the biological characteristics of these viruses that is relevant to their potential use as systemic oncolytic agents. Safety and efficacy of Ad6 and Ad11 suggest that development of these Ad serotypes for cancer treatment might be of particular value.
Materials and Methods
Cell lines and viruses
Human prostate cancer cell lines PC3, DU145, and LNCaP; human breast cancer cell lines SKBr-3, MDA-MB-468, and MDA-MB-231; human ovarian cancer cell lines SKOV-3 and OVCAR-3, human hepatocellular carcinoma cell lines Hep3B and HepG2, and human adenocarcinoma A459 were purchased from American Type Culture Collection (ATCC). All cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; HyClone). Wt Ad5, Ad6, Ad11, and Ad35 were obtained from ATCC. Suspension KB cells were maintained in HyQ MEM/EBSS (HyClone) supplemented with 10% FBS and were used for propagation of the viruses. Double CsCl banding was used for virus purification and vp concentration was determined by OD260 measurements.
Cell culture studies
Cells were infected with Ad serotypes at various MOIs and cell viability was measured by MTT assay (Sigma-Aldrich) on day 5 after the infection. To measure virus burst size, cells in 6-well plates were infected with Ads at MOI of 100 vp/cell for 2 hours in FBS-free medium. Then cells were washed three times and fresh FBS-containing medium was added to the cells. Cells and supernatants were collected at days 0 (2 hrs), 1, 3, 5, and 7 after the infection, freeze/thawed three times and titered on A549 cells by limiting dilution assay (n=3). Twenty-eight days later, the plates were read and the yields were divided by the initial number of cells to determine virus burst size. For factor X binding experiment, Ads were incubated with factor X (Haematologic Technologies) for 10 min at 37°C and then added to the cells. Final concentration of factor X was at physiological level (10 µg/ml). Three days later, cell viability was determined by MTT assay.
Flow cytometry
To determine CAR and CD46 expression profiles, cells were collected with the cell dissociation buffer (Invitrogen Corporation), stained with first Ab against CAR (Upstate) or CD46 (BD Pharmingen), or with isotype IgG1 control Ab (BD Pharmingen). The secondary Ab was goat anti-mouse IgG conjugated to R-phycoerythrin (Invitrogen). Ten thousand cells for each cell line were analyzed by flow cytometry.
Animals
Outbred ICR female mice (6–9 weeks old) were purchased from National Cancer Institute. Nude mice (4–6 weeks old) were purchased from Harlan Sprague Dawley. CD46-transgenic C57BL/6 mice (4–6 weeks old) were kindly provided by Roberto Cattaneo (Mayo Clinic).
Bioluminescence imaging
Mice were anesthetized with ketamine and xylazine and injected intraperitoneally with 100 µl of D-Luciferin (20 mg/ml) (Molecular Imaging Products). Imaged were taken by Kodak In-Vivo F system (Kodak) and processed and analyzed using Kodak Imaging Software (Kodak).
Liver toxicity studies
CD46-transgenic C57BL/6 mice were injected intravenously via the tail vein with 1.5×1011 vp of Ad5, Ad6, Ad11, or Ad35 (n=3) in 100 µl volume on day 0. Blood was collected at day 3 and analyzed for serum levels of alanine aminotransferase (ALT) by colorimetric endpoint reaction method according to manufacturer’s instructions (Biotron Diagnostics).
In vivo anticancer activity
DU145 tumors were established by injecting nude mice subcutaneously with 1×106 DU145 cells in 200 µl of growth medium containing 50% Matrigel (BD Biosciences). When average tumor volume reached 212 or 345 mm3 (25–54 days after cell injection), mice received single intratumoral injection of 100 µl of buffer or 3×1010 vp of Ads or two intravenous injections of 3×1010 vp separated by 4 hours in 100 µl volume. Tumor dimensions were measured twice a week with calipers and tumor volumes were calculated as width2×length×1/2. Mice were euthanized when the tumor volume reached 2000 mm3.
Statistical analyses
The data were fitted with sigmoid curves and EC50 values were calculated using GraphPad Prism 4 software. Statistical analyses were performed using SPSS software. The statistical significance was assessed with factorial ANOVA (in vitro data and in vivo toxicity data) or repeated measures ANOVA (tumor volume and predosing with Ad serotypes data) followed by Tukey’s HSD test for pair-wise comparisons between groups. Effect of factor X on in vitro activity of Ads was analyzed by 2-tailed unpaired t-test. Survival data were analyzed using the log rank test. P≤0.05 was considered significant.
ACKNOWLEDGMENTS
We thank Konstantin Doronin for valuable suggestions and Mary Barry and DeAnn Frederixon for helpful technical assistance. This work was supported by the NIH P50 CA91956 Prostate Cancer SPORE grant to Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J.Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikary AK, Banik U, Numaga J, Suzuki E, Inada T, Okabe N. Heterogeneity of the fibre sequence in subgenus C adenoviruses. J.Clin.Pathol. 2004;57:612–617. doi: 10.1136/jcp.2003.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M, Seely J, Kim JH, Freytag SO. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol.Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr.Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J.Virol. 2006;80:1688–1699. doi: 10.1128/JVI.80.4.1688-1699.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat.Rev.Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu DC, Charlton D, Henderson DR. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum.Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- Demers GW, Johnson DE, Tsai V, Wen SF, Quijano E, Machemer T, Philopena J, Ramachandra M, Howe JA, Shabram P, Ralston R, Engler H. Pharmacologic indicators of antitumor efficacy for oncolytic virotherapy. Cancer Res. 2003;63:4003–4008. [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J.Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat.Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J.Natl.Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Garnett CT, Talekar G, Mahr JA, Huang W, Zhang Y, Ornelles DA, Gooding LR. Latent species C adenoviruses in human tonsil tissues. J.Virol. 2009;83:2417–2428. doi: 10.1128/JVI.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterman L, Vogels R, van dV, Sieuwerts M, Grimbergen J, Kaspers J, Geelen E, van der HE, Lemckert A, Gillissen G, Verhaagh S, Custers J, Zuijdgeest D, Berkhout B, Bakker M, Quax P, Goudsmit J, Havenga M. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J.Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc.Natl.Acad.Sci.U.S.A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev.Med.Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, Thorne S, Reid T, Ni S, Lieber A, Fisher K, Seymour L, Rubanyi GM, Harkins RN, Hermiston TW. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS.ONE. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, Crystal RG. Intracellular trafficking of adenovirus: many means to many ends. Adv.Drug Deliv.Rev. 2007;59:810–821. doi: 10.1016/j.addr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin alpha(v)beta1 is an adenovirus coreceptor. J.Virol. 2001;75:5405–5409. doi: 10.1128/JVI.75.11.5405-5409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY. Differential expression of cell surface molecules in prostate cancer cells. Cancer Res. 2000;60:3429–3434. [PubMed] [Google Scholar]
- Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, Byrnes AP. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol.Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J.Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin.Diagn.Lab Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DE, Sandberg EM, Kyle MM, Devi NS, Brat DJ, Xu Z, Tighiouart M, Van Meir EG. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67:6872–6881. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW, Brough DE, Kovesdi I, Wickham TJ. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J.Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux JJ, Burnett RM. Adenovirus structure. Hum.Gene Ther. 2004;15:1167–1176. doi: 10.1089/hum.2004.15.1167. [DOI] [PubMed] [Google Scholar]
- Sakurai F, Kawabata K, Koizumi N, Inoue N, Okabe M, Yamaguchi T, Hayakawa T, Mizuguchi H. Adenovirus serotype 35 vector-mediated transduction into human CD46-transgenic mice. Gene Ther. 2006;13:1118–1126. doi: 10.1038/sj.gt.3302749. [DOI] [PubMed] [Google Scholar]
- Sakurai F, Nakamura S, Akitomo K, Shibata H, Terao K, Kawabata K, Hayakawa T, Mizuguchi H. Transduction properties of adenovirus serotype 35 vectors after intravenous administration into nonhuman primates. Mol.Ther. 2008;16:726–733. doi: 10.1038/mt.2008.19. [DOI] [PubMed] [Google Scholar]
- Sandberg L, Papareddy P, Silver J, Bergh A, Mei YF. Replication-competent Ad11p vector (RCAd11p) efficiently transduces and replicates in hormone-refractory metastatic prostate cancer cells. Hum.Gene Ther. 2009;20:361–373. doi: 10.1089/hum.2007.124. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Wigand R, Heinrich W. Worldwide epidemiology of human adenovirus infections. Am.J.Epidemiol. 1983;117:455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J.Virol. 2003;77:9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshidhar RP, Ganesh S, Limbach MP, Brann T, Pinkstaff A, Kaloss M, Kaleko M, Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Shashkova EV, Doronin K, Senac JS, Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008a;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008b;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova EV, Spencer JF, Wold WS, Doronin K. Targeting Interferon-alpha Increases Antitumor Efficacy and Reduces Hepatotoxicity of E1A-mutated Spread-enhanced Oncolytic Adenovirus. Mol.Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J.Virol. 2000;74:10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, Yu DC, Aimi J, Ando D, Working P, Kirn D, Wilding G. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol.Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Smith JS, Xu Z, Tian J, Stevenson SC, Byrnes AP. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum.Gene Ther. 2008;19:547–554. doi: 10.1089/hum.2008.004. [DOI] [PubMed] [Google Scholar]
- Stone D, Liu Y, Li ZY, Tuve S, Strauss R, Lieber A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol.Ther. 2007;15:2146–2153. doi: 10.1038/sj.mt.6300319. [DOI] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol.Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Tuve S, Wang H, Jacobs JD, Yumul RC, Smith DF, Lieber A. Role of cellular heparan sulfate proteoglycans in infection of human adenovirus serotype 3 and 35. PLoS.Pathog. 2008;4:e1000189. doi: 10.1371/journal.ppat.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuve S, Wang H, Ware C, Liu Y, Gaggar A, Bernt K, Shayakhmetov D, Li Z, Strauss R, Stone D, Lieber A. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J.Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, Tordjmann T, Vigne E, Perricaudet M, Benihoud K. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol.Ther. 2008;16:1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J.Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum.Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J.Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]