Figure 2. Binding of nsp1 to the 40S ribosomal subunits.
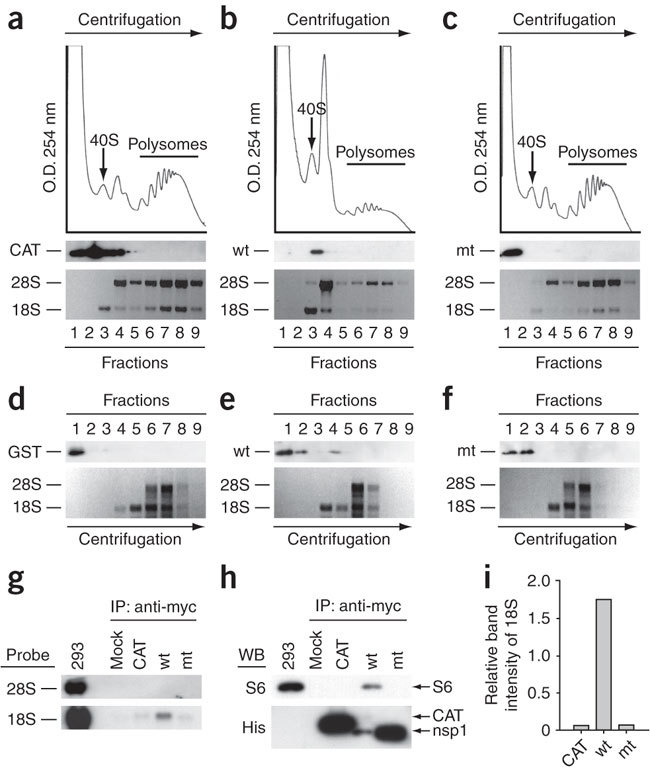
(a–c) CAT (a), nsp1 (b) or nsp1-mt (c) RNAs were transfected into 293 cells. Cell extracts were prepared 8 h after transfection and subjected to polysome profile analysis (top panels). CAT, nsp1 (wt) and nsp1-mt (mt) in each fraction were detected by western blot analysis using antibody to myc (middle panels). Bottom panels show rRNAs in each fraction. (d–f) After incubation of the mixture of 0.25 μg of rLuc RNA and 1 μg of nsp1, GST or nsp1-mt in RRL for 30 min at 30 °C, the samples were separated on 10%–50% sucrose gradient. GST, nsp1 (wt) and nsp1-mt (mt) in each fraction were detected by western blot analysis using anti-GST antibody and anti-nsp1 (ref. 8) antibody. The locations of GST (d), nsp1 (e) or nsp1-mt (f) in fractions are shown (all top panels). The bottom panels show rRNAs. (g–i) 293 cells were transfected with CAT RNA (CAT), nsp1 RNA (wt), or nsp1-mt RNA (mt). Cell extracts were prepared 8 h after transfection and subjected to immunoprecipitation using anti-myc antibody. Extracted RNAs from the immunoprecipitates were separated by agarose gel electrophoresis, and rRNAs were detected by northern blot analysis (g). The immunoprecipitated proteins were examined by western blot analysis using anti-S6 antibody (40S subunit specific) and anti-His antibody for CAT, nsp1 and nsp1-mt (h). Panel I represents the values, each of which was obtained by dividing the abundance of the immunoprecipitated 18S rRNA with the immunoprecipitated CAT protein, nsp1 protein or nsp1-mt protein in an arbitrary scale.
