Abstract
Little is known about the risk of recurrence more than five years after diagnosis among older breast cancer survivors. A community-based population of women ≥65 years diagnosed with early stage breast cancer who survived disease-free for five years was followed for five additional years or until a diagnosis of breast cancer recurrence, second primary, death or loss to follow-up. These five-year disease-free survivors (N=1277) had primary breast cancers that were node negative (77%) and estrogen receptor positive or unknown (86%). Five percent (N=61) developed a recurrence between five and ten years after diagnosis; 25% local, 9.8% regional, and 66% distant. Women who were node positive (Hazard Ratio [HR]=3.9; 95% Confidence Interval [CI]=1.5,10); had poorly differentiated tumors (HR=2.5; 95% CI=0.9, 6.6); or who received breast conserving surgery without radiation therapy (HR=2.4; 95%CI=1.0, 5.8) had higher recurrence rates compared with node negative, well differentiated, and receipt of mastectomy, respectively. Not receiving adjuvant tamoxifen, compared with receiving adjuvant tamoxifen, was also positively associated with late recurrence among women with estrogen receptor positive/unknown tumors. While relatively few women experience a late recurrence, most recurrences present as advanced disease, which is difficult to treat in older women. This study of late recurrence emphasizes that the risk, although small, is not negligible even in this group at high risk for death due to competing causes.
Keywords: Breast cancer, recurrence, primary therapy, cancer survivorship, older women
Introduction
Survivorship is defined as the time between cancer diagnosis and death (1). Currently, there are over 2.6 million breast cancer survivors in the United States and 1.6 million of them are ≥65 years old.1 As the number of breast cancer survivors increases due to improvements in screening, primary treatment, and adjuvant therapy (1), identifying factors associated with survival after five years becomes more important. Older women have the highest incidence and prevalence of breast cancer (2), yet little is known about factors affecting the risk of recurrence among women who remain disease-free five years after diagnosis. Recently, Brewster et al observed higher stage (stage II versus stage I) and hormone receptor positive tumors (versus hormone receptor negative tumors) without hormonal therapy were associated with risk of recurrence after five years (3). However, the majority of the population (78%) was <60 years old and from one tertiary care cancer center (3), leaving an important gap regarding late recurrence risk in older women, who are the majority of breast cancer survivors. The objective of this study is to describe the risk for, and identify predictors of, breast cancer recurrence beyond five years in the community-based population of breast cancer survivors ≥65 years old who were included in the Breast Cancer Treatment Effectiveness in Older Women (BOW) cohort (4).
Materials and Methods
The BOW cohort (4, 5) includes women ≥65 years old at diagnosis with stage I or II breast cancer from 1990 to 1994 in six geographically diverse Cancer Research Network (CRN) (6) healthcare systems: Group Health, Seattle, Washington; Fallon Clinic, Worcester, Massachusetts; Kaiser Permanente Southern California, Pasadena, California; Lovelace Health System, New Mexico; HealthPartners, Minneapolis, Minnesota; and Henry Ford Health System, Detroit, Michigan. Through collaborative research, the CRN aims to increase effective preventive, curative, and supportive interventions for major cancers among diverse populations and health systems. Women who had any cancer diagnosed within five years before their incident breast cancer diagnosis were excluded. We chose five years for this boundary because cancers diagnosed more than five years earlier would be rare and would not affect breast cancer treatments or outcomes. The institutional review boards of the six participating healthcare systems and Boston University Medical Center approved this study.
Demographic characteristics, breast cancer tumor characteristics and treatments, comorbid conditions, and breast cancer recurrence were collected from tumor registries, administrative data, and medical record review (7). The inter-rater reliability of medical record abstraction was ≥ 90% overall (8); with 90% sensitivity and 96% specificity for breast cancer recurrence classification.
The survivor population for the reported study included women from the BOW cohort who survived disease-free for five years after initial breast cancer diagnosis (Figure 1). Follow-up began five years after diagnosis and accrued until the diagnosis of first recurrence, second primary, death from any cause, disenrollment from the healthcare system, or survival for an additional five years (ten years disease-free from diagnosis), whichever came first.
Figure 1.
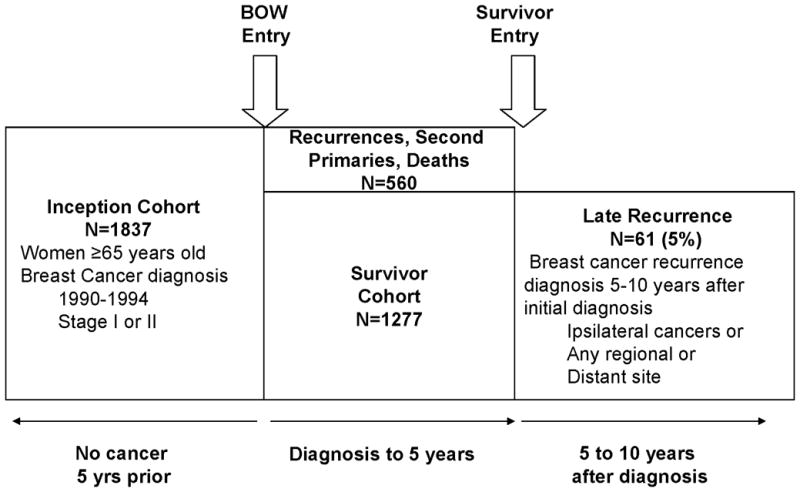
Inclusion criteria for the BOW inception cohort, five-year disease-free survivor cohort, and women diagnosed with a late recurrence in a population of older breast caner survivors.
Late breast cancer recurrence was defined as a tumor that was pathologically or clinically diagnosed between five and ten years after diagnosis in the same breast as the original tumor or in any regional or distant site (Figure 1). A tumor that occurred in the contralateral breast (second primary) was not considered a late recurrence because primary tumor therapy is aimed at reducing the risk of recurrence, not occurrence of disease in the contralateral breast. Therefore, women were censored at the date of diagnosis of second primary breast cancer.
We included age at diagnosis (65–69; 70–79; ≥80), race/ethnicity (non-Hispanic White; Hispanic and/or Other Race), Charlson Comorbidity Index (9) in the year before diagnosis (0; 1; ≥2),tumor size (<1; 1-<2; 2-<3; ≥4 centimeters), nodal involvement (0; 1–3; ≥4), histologic grade (well differentiated; intermediate/moderate differentiated; poorly differentiated/undifferentiated/anaplastic), primary therapy (mastectomy; breast conserving surgery (BCS) plus radiation therapy (RT); BCS only), progesterone receptor expression (positive or unknown; negative), estrogen receptor (ER) expression (positive or unknown; negative), receipt of adjuvant tamoxifen (yes; no), and receipt of adjuvant chemotherapy (yes; no) as potential predictors of late recurrence.
The roles of estrogen and progesterone receptors in recurrence risk, and how they impact the effectiveness of tamoxifen, present an analytic challenge. Progesterone receptor expression has little impact on the protective effect of tamoxifen after accounting for ER expression, whereas the protective effect of tamoxifen in women with unknown ER expression is similar, although weaker in magnitude, to its effect in women with ER-positive tumors (10). Our results for all combinations of tamoxifen use (yes; no) cross-tabulated by estrogen and progesterone receptor expression together and separately, including and excluding women with unknown hormone expression, were all similar. We report the results of the model adjusting for a four category variable created by cross-tabulating ER-positive/unkown expression by receipt of adjuvant tamoxifen.
We described tumor and treatment characteristics using univariate statistics. Using the survivor cohort, all of the predictors of recurrence after five years were included in a Cox proportional hazards regression model using SAS version 9.1 (Cary, North Carolina). Since breast cancer-specific mortality is often used as a surrogate outcome for recurrence, we also evaluated predictors of breast cancer-specific mortality among the five-year disease free survivors, following methods described earlier to identify deaths from breast cancer (11).
Results
In Table 1, we provide characteristics of women who had definitive surgery from the BOW cohort at its inception (N=1837) to put the characteristics of women who survived disease-free for five years (N=1277) in context. We also show descriptive characteristics for the women who experienced a late recurrence (N=61). Similar to the BOW inception cohort, most of the five-year disease-free survivors were between 65 to 74 years old at diagnosis (67%), non-Hispanic white (81%), had no nodal involvement (77%), and had tumors with ER-positive (or unknown) expression (86%). Among survivors with ER-positive (or unknown) expression, 32% were not prescribed, 39% received less than five years, and 29% received five years or more of adjuvant tamoxifen. Women with ER-positive (or unknown) tumors who did not receive tamoxifen had less aggressive tumor characteristics than women with ER-positive (or unknown) expression who received tamoxifen (tumors ≤2 centimeters [85% versus 45%, respectively], no nodal involvement [98% versus 68% respectively], and well/moderately differentiated tumors [64% versus 38% respectively]) (data not shown).
Table 1.
Descriptive, tumor, and treatment characteristics for older women of the inception cohort* who received surgery (n=1837), five-year disease-free breast cancer survivors (n=1277), and survivors who developed a recurrence between five and ten years after diagnosis (n=61).
| Characteristic | Inception Cohort (n=1837) | 5 Year Survivor Cohort (n=1277) | Recurrence five to ten years after diagnosis (n=61) | HR†(95% CI) | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age categories | |||||||
| 65–69 | 626 | 34 | 478 | 37 | 24 | 39 | Reference |
| 70–74 | 547 | 30 | 388 | 30 | 26 | 43 | 1.7 (0.9, 2.9) |
| 75–79 | 304 | 17 | 201 | 16 | 6 | 9.8 | 0.8 (0.3, 2.0) |
| ≥80 | 360 | 20 | 210 | 16 | 5 | 8.2 | 0.8 (0.3, 2.3) |
| Race/Ethnicity | |||||||
| Non-Hispanic White | 1503 | 82 | 1036 | 81 | 46 | 75 | Reference |
| Hispanic and/or Other Race | 334 | 18 | 241 | 19 | 15 | 25 | 1.2 (0.7, 2.5)‡ |
| Charlson Comorbidity Index | |||||||
| 0 | 1252 | 68 | 926 | 73 | 44 | 72 | Reference |
| 1 | 498 | 27 | 307 | 24 | 16 | 26 | 1.4 (0.7, 2.5) |
| ≥2 | 87 | 4.7 | 44 | 3.5 | 1 | 1.6 | 0.6 (0.1, 4.4) |
| Tumor Size | |||||||
| <1 cm | 376 | 20 | 295 | 23 | 11 | 18 | Reference |
| 1 to < 2 cm | 759 | 41 | 548 | 43 | 23 | 38 | 1.1 (0.5, 2.3) |
| 2 to < 3 cm | 428 | 23 | 278 | 22 | 16 | 26 | 1.2 (0.5, 2.8) |
| ≥3 cm | 274 | 15 | 156 | 12 | 11 | 18 | 1.3 (0.5, 3.3) |
| Node positivity | |||||||
| 0 nodes | 1371 | 75 | 984 | 77 | 30 | 49 | Reference |
| 1 to 3 nodes | 333 | 18 | 236 | 18 | 23 | 38 | 2.5 (1.3, 4.8) |
| ≥4 nodes | 133 | 7.2 | 57 | 4.5 | 8 | 13 | 3.9 (1.5, 10.1) |
| Histologic Grade | |||||||
| Well differentiated | 297 | 16 | 238 | 19 | 6 | 9.8 | Reference |
| Intermediate/moderate | 676 | 37 | 473 | 37 | 25 | 41 | 1.9 (0.8, 4.7) |
| Poorly differentiated/undifferentiated/anaplastic | 429 | 23 | 266 | 21 | 22 | 36 | 2.5 (0.9, 6.6) |
| Not determined/stated | 435 | 24 | 300 | 23 | 8 | 13 | 1.1 (0.4, 3.1) |
| Primary Therapy | |||||||
| Mastectomy | 977 | 53 | 658 | 52 | 32 | 52 | Reference |
| Breast Conserving Surgery (BCS) plus Radiation Therapy | 639 | 35 | 499 | 39 | 22 | 36 | 1.4 (0.8, 2.7) |
| BCS | 221 | 12 | 120 | 9.4 | 7 | 11 | 2.4 (1.0, 5.8) |
| Progesterone Receptor Expression | |||||||
| Positive or Unknown | 1538 | 84 | 1093 | 86 | 52 | 85 | Reference |
| Negative | 299 | 16 | 184 | 14 | 9 | 15 | 0.6 (0.3, 1.1) |
| Estrogen Receptor Expression | |||||||
| Positive or Unknown | 1538 | 84 | 1093 | 86 | 52 | 85 | |
| Received Tamoxifen | 1028 | 67 | 746 | 68 | 42 | 81 | Reference§ |
| No Tamoxifen | 510 | 33 | 347 | 32 | 10 | 19 | 2.4 (0.7, 8.1) |
| Negative | 299 | 16 | 184 | 14 | 9 | 15 | |
| Received Tamoxifen | 134 | 45 | 75 | 41 | 3 | 33 | 0.5 (0.1, 2.2) |
| No Tamoxifen | 165 | 55 | 109 | 59 | 6 | 67 | 1.6 (0.5, 4.4) |
| Chemotherapy received | |||||||
| Yes | 187 | 10 | 120 | 9.4 | 14 | 23 | 1.2 (0.5, 2.7) |
| No | 1243 | 90 | 1157 | 91 | 47 | 77 | Reference |
The inception cohort is similar to that reported by Geiger et al.5 The variables have been modified by collapsing categories and recategorizing race/ethnicity, tumor size, node positivity, progesterone receptor expression, estrogen receptor expression and tamoxifen therapy. The inception cohort was not used in any analyses for late recurrence.
Cox proportional hazards model simultaneously included all predictors and health care system.
Non-Hispanic White compared to Hispanic and/or Other Race (African American or Asian).
The reference group for the comparison of positive or unknown ER expression compared with negative ER expression.
The rate of recurrence was higher in the first five-years (40 per 100 woman-years) than the second five-years (26 per 100 woman-years). Five percent (N=61) of women disease-free at five-years had a recurrence between five and ten years after diagnosis (late recurrences). Of the women with a late recurrence, 25% presented with local disease, 9.8% with regional disease, and 66% with distant metastases. The median time to recurrence after the first five disease-free years was 2.8 years.
Women who had four or more positive nodes at their original diagnosis had nearly four times the rate of late recurrence compared with node negative women. Women with poorly differentiated tumors had 2.5 times the recurrence rate as women with well-differentiated tumors. When tumor size and nodal involvement were combined to form stage categories, women with stage II tumors had 2.6 times the recurrence rate as stage I women (95%CI =1.4, 5.0). Higher recurrence rates were also experienced by women who received BCS without RT than women who received a mastectomy. For adjuvant therapy, there appeared to be an increased rate of late recurrence among the women with ER-positive (and unknown) tumors who did not receive adjuvant tamoxifen compared with women who received tamoxifen. However we did not observe an association between receipt of adjuvant chemotherapy and late recurrence (Table 1).
The results for breast cancer-specific mortality parallel those for late recurrence. Women with four or more positive nodes (HR=4.5; 95%CI=1.9, 11) and who received BCS without RT (HR=2.1; 95%CI=1.1, 4.3) were more likely to die of breast cancer five to ten years after diagnosis compared with women who were node negative or received a mastectomy, respectively.
Discussion
In a large cohort study of older women with early-stage breast cancer treated in community-based settings, we found that tumor characteristics and primary tumor therapy continue to predict recurrence risk after five years of disease-free survival. Specifically we found that nodal involvement, poor histology, and breast conserving surgery without radiation therapy increased the rate of late recurrence. We also observed a positive association between not receiving adjuvant tamoxifen and the risk of late recurrence among ER-positive women.
Our results are consistent with previous findings of mostly younger women (3, 11–14). Although comorbid diseases were not associated with late recurrence in our older cohort, existing comorbidities were associated with receipt of suboptimal treatment (4). More aggressive tumor characteristics and inadequate primary tumor therapy increased the risk of recurrence after five years (3, 12–15). The association between higher stage (stage II versus stage I) is similar to the finding of Brewster et al (3). The positive association among ER-positive women who did not receive adjuvant tamoxifen and late recurrence is also consistent with previous findings (12–15). However, we must be cautious in our interpretation of this finding since our data are also compatible with a 30% protective effect of adjuvant tamoxifen on late recurrence among ER-positive women.
The risk of recurrence among ER-positive women has been shown to vary over time with a greater risk of recurrence in the later years than in the first two years (12–15). Studies have shown that ER-negative tumors mostly recur within the first five years after diagnosis, while ER-positive tumors recur later (13, 14). In addition, early discontinuation of adjuvant tamoxifen before the completion of five years may also explain our increased risk of recurrence among our ER-positive women (12). Approximately 70% of our older survivors with ER-positive (and unknown) tumors received adjuvant tamoxifen. However, nearly 40% received less than five years of adjuvant tamoxifen. These women may not have had the complete benefit of tamoxifen because they were treated in the 1990s when recommendations for the duration of tamoxifen treatment were evolving, and before the advent of aromatase inhibitors (16, 17). Although there is growing support for our finding (3, 12–14), the biological mechanisms explaining the difference in risk over time by ER expression are still unknown. At the time of diagnosis and treatment of our cohort CYP2D6 and subtypes of ER-positive expression (human epidermal growth factor receptor 2 [Her2]) were not routinely assayed. Future evaluation of late breast cancer recurrence by genetic factors and subtypes may be useful to further explain the increased risk beyond five years in older women with ER-positive tumors (18, 19).
Although treatment patterns of today differ from the 1990s, ten years of follow-up requires that women were diagnosed with incident breast cancer in the 1990s. An additional limitation is that the majority of our older survivor cohort was non-Hispanic White, so our results may not apply to risk in other racial and ethnic populations. Despite these limitations, the BOW cohort is one of the largest cohorts of older women with breast cancer in the United States and has the unique feature of nearly complete follow-up through ten years.
To our knowledge, this study is one of the first to specifically assess the risk of late recurrence in a cohort restricted to older women. Older women with breast cancer increasingly survive five years or more after initial diagnosis, despite their risk for death due to breast cancer and other competing causes. These women remain at risk for recurrence as well as coexisting morbidity and mortality five to ten years after their diagnosis. While relatively few older women recur after five years, those who do often have advanced cancers that are difficult to treat. To improve outcomes for older women, particularly those with a five-year or longer life expectancy, providing guideline therapy for the primary tumor (20, 21) and surveillance for recurrence beyond five years (22) of survival are essential to reducing morbidity and mortality, and maintaining quality of life.
Footnotes
Cancer Survivorship Research [Internet]. Rockville: National Cancer Institute, Division of Cancer Control and Population Sciences [updated 2006 Nov 6; cited 2009 Jun 11]. Available from: http://cancercontrol.cancer.gov/ocs/definitions.html
References
- 1.Berry DA, Cronin KA, Plevritis SK, et al. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. N Engl J Med. 2005;353:1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.Lash TL, Silliman RA. Re: prevalence of cancer. J Natl Cancer Inst. 1998;90:399–400. doi: 10.1093/jnci/90.5.399. [DOI] [PubMed] [Google Scholar]
- 3.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual Risk of Breast Cancer Recurrence 5 Years After Adjuvant Therapy. J Natl Cancer Inst. 2008;100:1179–83. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enger SM, Thwin SS, Buist DSM, et al. Breast Cancer Treatment of Older Women in Integrated Health Care Settings. J Clin Oncol. 2006;24:4377–83. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiger A, Thwin S, Lash T, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–74. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EH, Greene SM, Hart G, et al. Building a Research Consortium of Large Health Systems: The Cancer Research Network. J Natl Cancer Inst Monogr. 2005;2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 7.Thwin S, Clough-Gorr K, McCarty M, et al. Automated inter-rater reliability assessment and electronic data collection in a multi-center breast cancer study. BMC Med Res Methodol. 2007;7:23. doi: 10.1186/1471-2288-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lash TL, Fox MP, Thwin SS, et al. Using probabilistic corrections to account for abstractor agreement in medical record reviews. Am J Epidemiol. 2007;165:1454–61. doi: 10.1093/aje/kwm034. [DOI] [PubMed] [Google Scholar]
- 9.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaboration Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Ulcickas Yood M, Owusu C, Buist DSM, Geiger AM, Field TS, Thwin SS, Lash TL, Prout MN, Frost FT, Enger SM, Silliman RA. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Natarajan L, Pu M, Parker BA, et al. Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. Am J Epidemiol. 2009;169:1463–70. doi: 10.1093/aje/kwp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess KR, Puszati L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Research Treat. 2003;78:105–18. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 14.Dignam JJ, Dukic V, Anderson SJ, Mamounas EP, Wickerham DL, Wolmark N. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Research Treat. 2009 doi: 10.1007/s10549-008-0200-5. published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Research Treat. 1998;52:227–37. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- 16.Winer E, Gralow J, Diller L, et al. Clinical Cancer Advances 2008: Major Research Advances in Cancer Treatment, Prevention, and Screening--A Report from the American Society of Clinical Oncology. J Clin Oncol. 2009;27(5):812–826. doi: 10.1200/JCO.2008.21.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seruga B, Tannock IF. Up-Front Use of Aromatase Inhibitors As Adjuvant Therapy for Breast Cancer: The Emperor Has No Clothes. J Clin Oncol. 2009;27(6):840–842. doi: 10.1200/JCO.2008.19.5594. [DOI] [PubMed] [Google Scholar]
- 18.Punglia RS, Burstein HJ, Winer EP, Weeks JC. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Nat Cancer Instit. 2008;100:642–8. doi: 10.1093/jnci/djn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 20.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–5. [PubMed] [Google Scholar]
- 21.Glick JH, Gelber RD, Goldhirsch A, Senn H-J. Meeting Highlights: Adjuvant Therapy for Primary Breast Cancer. J Natl Cancer Inst. 1992;84:1479–85. doi: 10.1093/jnci/84.19.1479. [DOI] [PubMed] [Google Scholar]
- 22.Lash TL, Fox MP, Buist DSM, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–6. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]


