Figure 4.
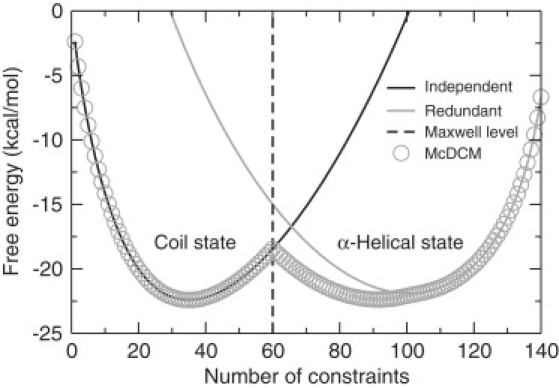
One-dimensional free energy landscapes at fixed temperature (T = 302 K) are shown as a function of number of constraints. All curves were generated with McDCM parameters for the A6 polypeptide. The (black, gray) curves show free energy landscapes with a single basin centered on the (left, right) side when all constraints are modeled as either (independent, redundant). Open circles show a free energy landscape with a double basin predicted by the McDCM, indicating cooperativity arises from a competition between microstates that are primarily flexible in the coil state and rigid in the α-helical state. The vertical dash-dotted line denotes the Maxwell level (i.e., the number of constraints needed to make the polypeptide just rigid), which indicates the polypeptide is globally (flexible, rigid) to its (left, right).
