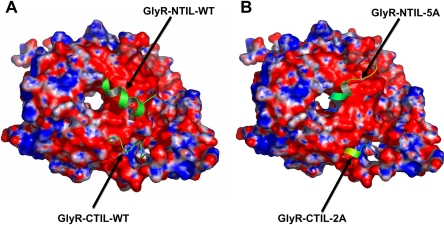
Fig. 3.
Molecular docking of N- and C-terminal fragments to Gβγ. Molecular docking was performed using crystallographic three-dimensional data of Gβγ (blue and red represent regions of positive and negative electrostatic potential, respectively) as described under Materials and Methods. A, WT N- and C-terminal fragments (Arg309–Lys325 and Arg382–Arg392) interact with high ΔG binding in different regions on the highly electronegative surface of Gβγ. B, mutant N- and C-terminal fragments [316-320A (5A) and 385-386A (2A)] interact with Gβγ with a reduced ΔG binding (see text).