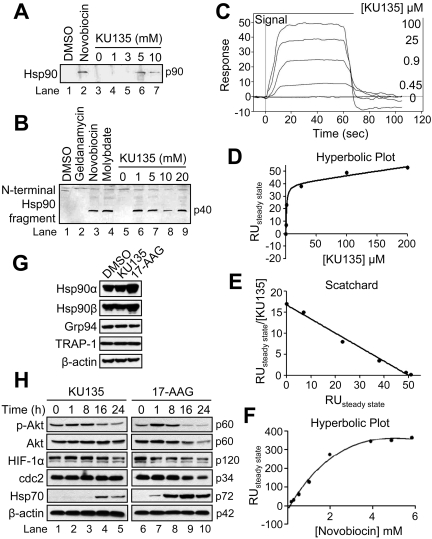
Fig. 3.
KU135 binds Hsp90 and exhibits effects different from 17-AAG on the expression levels of Hsp90 isoforms and known client proteins. A, rabbit reticulocyte lysate (50 μl) was applied to a novobiocin-Sepharose column after ATP regeneration and eluted with the indicated concentrations of KU135 and Western-blotted. DMSO and 10 mM novobiocin were used as controls. B, rabbit reticulocyte lysate (50 μl) was incubated at 30°C for 10 min with DMSO, 20 mM geldanamycin, novobiocin and molybdate (lanes 1–4, respectively) or 0, 1, 5, 10, and 20 mM KU135 (lanes 5–9). Subsequently, the reactions were chilled on ice and incubated for 6 min with 125 μg/ml trypsin and Western blotted. C to F, representative curves of SPR analysis of KU135 binding to Hsp90β injected at the indicated concentrations (C), hyperbolic replot of the amount of KU135 bound versus concentration of KU135 (D), a Scatchard plot of the binding of KU135 to Hsp90 (E), and hyperbolic replot of the amount of novobiocin bound versus concentration of novobiocin, corrected for nonspecific binding as described under Materials and Methods (F). G and H, cells (5 × 105/ml) were cultured with DMSO, 1 μM KU135, or 10 μM 17-AAG for 24 h, harvested, and lysed for Western blotting. β-Actin was used as a loading control. RU, relative units.