Fig. 1.
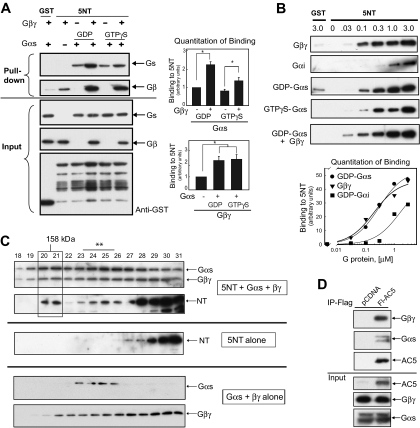
The N terminus of AC5 anchors heterotrimeric G proteins. A, GST or GST-tagged 5NT (final concentration, 2 μM) was incubated with G protein subunits Gαs (GDP or GTPγS bound) and/or Gβ1γ2 as indicated, and GST pull-down assay was performed. Western blot analysis indicated that 5NT binds GDP-Gαs and Gβγ independently and together their binding is enhanced (top). Protein input for Gαs-, Gβγ-, and GST-tagged proteins is shown below. Quantitation of binding for GDP-Gαs and Gβγ is shown (n = 3, P < 0.05). B, dose response of 5NT binding to Gβγ, GDP-Gαi, GDP-Gαs, GTPγS-Gαs, or GDP-Gαs in the presence of 100 nM Gβγ was performed by GST pull-down of 5NT (0.125 μM) and varying concentration of G protein subunits. Quantitation of binding from three independent experiments is shown (P < 0.05). C, gel filtration analysis of complex formation between 5NT and Gαs·βγ. Proteins (10 μM each) were applied on tandem Superdex 75/200 columns in buffer containing 0.05% C12E9 and 10 μM GDP and analyzed by SDS-PAGE and immunoblotting. Top, complex of 5NT/Gαs/Gβγ; middle, 5NT alone; bottom, Gαs/Gβγ. The 5NT/Gαs/Gβγ complex is boxed, whereas smaller complexes containing 5NT with Gαs or Gβγ are marked with an asterisk. D, IP of AC5-Gαs-Gβγ complex. Flag-tagged human AC5 (Fl-AC5) or pCDNA3 vector was transfected in HEK293 cells (10 cm dish/IP), immunoprecipitated with anti-Flag agarose, and subjected to Western blotting with anti-Gαs, anti-Gβ, or anti-Flag. The input represents 5% of the total used in the IP.