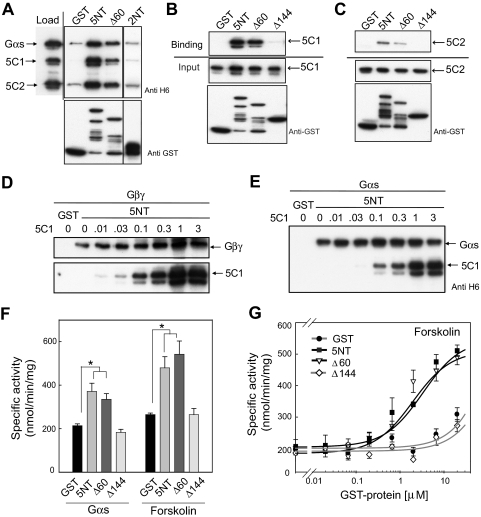
Fig. 3.
Interaction of 5NT with the catalytic core. A, 5NT pulls down 5C1/5C2/GTPγS-Gαs/forskolin complex. 5C1, 5C2, and GTPγS-Gαs (1 μM each) were incubated in presence of 100 μM forskolin for 30 min on ice before addition of GST, GST-5NT, or 2NT (2 μM). GST pull-down assay was performed as described under Materials and Methods. Western blot analysis of input and eluted proteins in shown. 5C1 (B) and 5C2 (C) were subjected to a GST pull-down assay with 5NT, Δ60, or Δ144 (2 μM). Western blot analysis of bound proteins and input is shown. Competition reactions between 5C1 (0–3 μM) and 200 nM Gβγ (D) or 200 nM Gαs-GDP (E) for binding to GST-5NT using GST pull-down assays. F, 5NT enhances the Gαs- or forskolin-stimulated activity of 5C1/5C2. Purified AC5 catalytic domains 5C1 (70 nM) and 5C2 (1 μM) were preincubated with GST or GST-tagged 5NT (5 μM) for 10 min before stimulation with either 400 nM GTPγS-GαS or 100 μM forskolin. G, dose-dependent enhancement of 5C1/5C2 activity by 5NT. AC activity assay was performed as described in F with varying concentrations of GST, 5NT, 5NTΔ60, or 5NTΔ144.