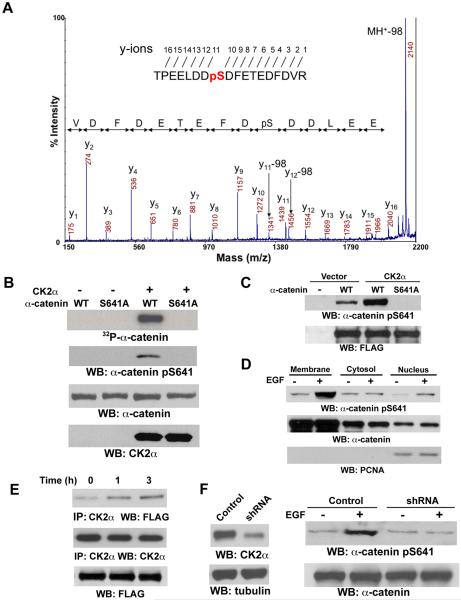
Figure 2. CK2α Interacts with and Phosphorylates α-catenin at S641.
(B-E) Immunoblotting analyses were performed with the indicated antibodies.
(A) α-catenin immunoprecipitated from EGF-stimulated A431 cells was analyzed with mass spectrometry. Mass spectrometric analysis of a tryptic fragment 634-TPEELDDSDFETEDFDVR-651 indicates S641 was phosphorylated. The m/z difference between y-11 and y-10 matched with phospho-Ser.
(B) In vitro kinase assays were performed with purified bacterially expressed WT His-α-catenin or His-α-catenin S641A with or without CK2α.
(C) pRc/CMV2-HA-CK2α was co-transfected with pEGFP N1-FLAG-tagged WT α-catenin or α-catenin S641A mutant into 293T cells. Immunoblotting of immunoprecipitated FLAG-tagged α-catenins with the anti–α-catenin Ser641 antibody was performed.
(D) The subcellular fractionation was prepared from A431 cells treated with EGF (100 ng/ml) for 30 min.
(E) A431 cells expressing FLAG-tagged WT α-catenin were treated with or without EGF (100 ng/ml) for 1 or 3 hr. CK2α was immunoprecipitated.
(F) A431 cells with or without expression of CK2α shRNA or a control shRNA were treated with or without EGF (100 ng/ml) for 30 min.