FIGURE 5.
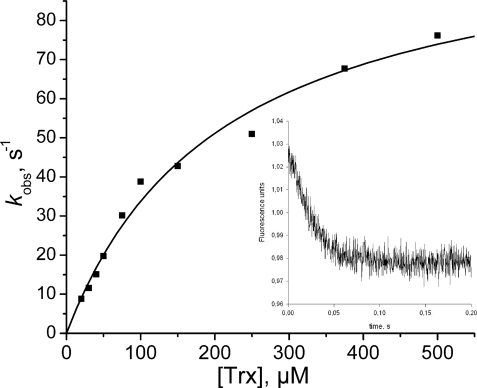
Kinetics of reduction of the Cys48/Cys84Srx disulfide bond by E. coli Trx. The Trx fluorescence quenching associated with disulfide bond formation was recorded on a stopped-flow apparatus at 30 °C in buffer TK by mixing reduced Trx and Cys48/Cys84-oxidized C106A Srx (10 μm). Excitation wavelength was set at 295 nm, and emitted light was collected using a 320 nm cutoff filter. Data collected at various concentrations of reduced Trx were fitted to Equation 1. The deduced kobs values plotted versus Trx concentration (■) were then fitted against Equation 2, which gave kobs, max and KS values of 105 ± 7 s−1 and 210 ± 30 μm (solid line). Inset, typical time course obtained for 250 μm Trx.