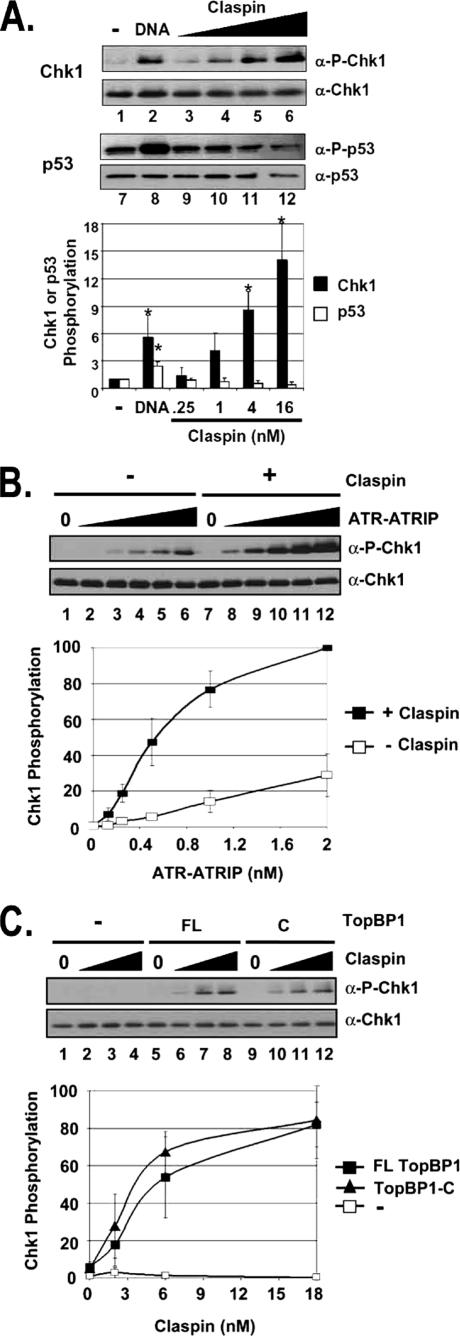
FIGURE 1.
Claspin specifically stimulates TopBP1-dependent ATR phosphorylation of Chk1 in a defined system. A, Claspin specifically stimulates ATR phosphorylation of Chk1 but not p53. All reactions contain 0.25 nm ATR and 0.5 nm TopBP1. Reactions 1–6 contain 10 nm His-Chk1kd, and reactions 7–12 contain 10 nm GST-p53. 1 ng of BPDE-modified 2-kb linear DNA was added to reactions in lanes 2 and 8. The values in lanes 1 and 7 are normalized to 1. ATR kinase activity was determined by immunoblotting for phospho-Chk1 (α-P-Chk1), Chk1, phospho-p53 (α-P-p53), and p53 as indicated. The graph shows quantitative analysis of the data from three independent experiments conducted under identical conditions. DNA stimulates both Chk1 and p53 phosphorylation, whereas Claspin stimulates Chk1 but not p53 phosphorylation. Values that are statistically different (T value <0.03 in the paired t test) than the control reactions (lanes 1 or 7) are indicated with an asterisk. The error bars indicate the average deviation from the mean. B, Claspin-mediated phosphorylation of Chk1 is dependent on ATR. 0, 0.125, 0.25, 0.5, 1, and 2 nm purified ATR-ATRIP was added to reactions lacking Claspin (lanes 1–6) or containing 16 nm Claspin (lanes 7–12) in addition to 0.5 nm TopBP1 and 10 nm Chk1kd. The results from three experiments were quantified and plotted. 0.25 nm ATR-ATRIP was chosen for the later kinase reactions. C, ATR activation by Claspin is dependent on TopBP1, and the C-terminal fragment of TopBP1 is sufficient. Kinase reactions containing 0, 2, 6, or 18 nm Claspin were performed without TopBP1 (lanes 1–4), with 1 nm full-length TopBP1 (FL) (lanes 5–8), or with 1 nm of the TopBP1-C fragment (C) (lanes 9–12).