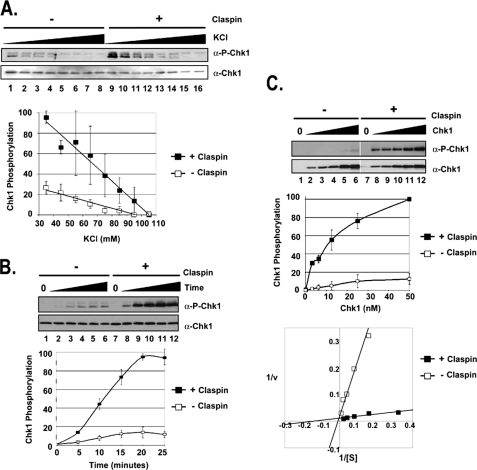
FIGURE 2.
Claspin mediates the phosphorylation of Chk1 by ATR through a salt-sensitive mechanism that increases the affinity of ATR for Chk1. A, Claspin stimulation of ATR phosphorylation of Chk1 is sensitive to ionic strength. Kinase assays were carried out with ATR-ATRIP (0.25 nm), Chk1 (10 nm), and TopBP1-C (0.5 nm) under different ionic strength conditions (35–105 mm KCl) in the absence or presence of 16 nm Claspin. The error bars indicate the average deviation from the mean. α-P-Chk1, phospho-Chk1; α-P-Chk1-p53, phospho-p53. B, kinetics of Claspin stimulation of phosphorylation of Chk1 by ATR. Kinase assays were performed as described in the legend for Fig. 1 with 10 nm His-Chk1 and incubated for the indicated amount of time without Claspin or with 16 nm Claspin. C, Claspin affects the affinity of ATR for Chk1. Kinase assays were performed as described in B, except that the Chk1 concentration varied from 0 to 50 nm. The results from three experiments were quantified and plotted as before (top graph) or with the Lineweaver-Burk format of 1/v versus 1/[S] (bottom graph), which allows for calculating the Km = −1/x-intercept and the Vmax = 1/y-intercept. The equation for the lines fitting the data for reactions lacking Claspin is y = 2.1049x + 0.0175, and for reactions containing Claspin, it is y = 0.0784x + 0.011. The units for Vmax have been determined by standardizing the signal from Western blotting to the amount of Pi incorporated in experiments containing radioactive [γ-32P]ATP. v, velocity.