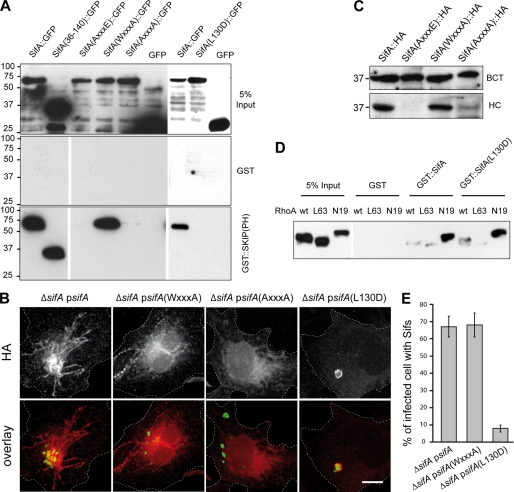
FIGURE 3.
Biochemical and functional analysis of point mutants of SifA: interaction with SKIP and RhoA, translocation, and formation of Sifs. A, pulldown analysis of the interaction between SKIP(PH) and SifA variants. GST::SKIP(PH) or GST were immobilized on beads and incubated with extracts of HeLa cells expressing SifA, SifA-(36–140), or SifA variants (AXXXE, WXXXA, AXXXA, and L130D) fused to the N terminus of GFP or GFP alone. Bound proteins were analyzed by Western blotting with an anti-GFP antibody. B and C, translocation analysis. HeLa cells were infected for 16 h with ΔsifA strains expressing GFP and 2HA-tagged version of wild-type or point-mutation variants of SifA. Cells were either fixed, immunolabeled for HA, and imaged by confocal microscopy for GFP (green) and HA (red) (scale bar, 10 μm) (B) or subjected to Triton X-100 extraction and differential centrifugation and analyzed by Western blotting for HA-tagged proteins in bacterial (BCT) and HeLa cell (HC) fractions (C). D, both SifA and SifA-(L130D) pull down GDP-bound RhoA. GST::SifA, GST::SifA-(L130D), or GST were immobilized on beads and incubated with extracts of HeLa cells expressing HA-tagged wild-type, GTP-bound (L63), or GDP-bound (N19) forms of RhoA. Pulled down proteins were analyzed by Western blotting with an anti-HA antibody. E, SifA-(L130D) does not support the formation of Sifs. HeLa cells were infected for 16 h, immunostained, and scored for the formation of HA-labeled Sifs.