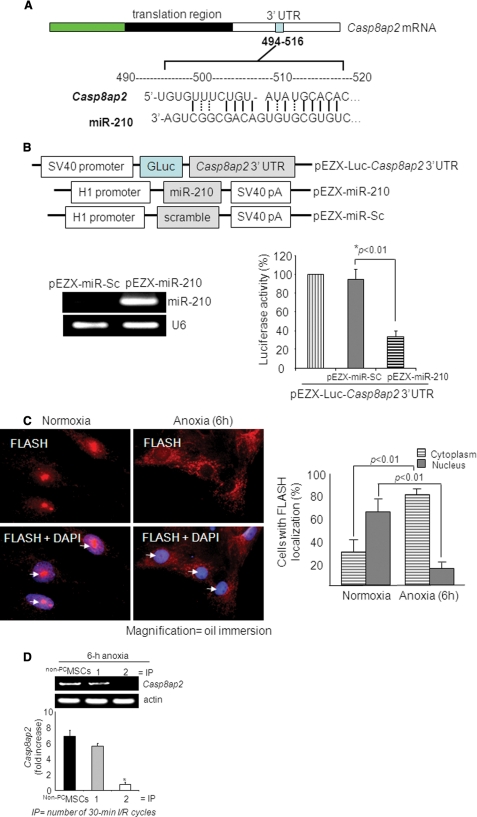
FIGURE 3.
miR-210 targets Casp8ap2, a positive regulator of apoptosis. A, one putative target site of miR-210 highly conserved in the Casp8ap2 mRNA 3′-UTR. B, construction of pEZX-Luc-Casp8ap2 3′-UTR luciferase reporter plasmid and precursor miR-210 expression clone. qRT-PCR showed successful transfection and significantly higher expression of miR-210 in MSCs using pEZX-miR-210 plasmid compared with pEZX-miR-Sc plasmid-transfected MSCs. Cotransfection of MSCs with pEZX-Luc vector containing Casp8ap2 3′-UTR together with a plasmid encoding miR-210 showed decreased luciferase activity (p < 0.01 versus pEZX-miR-Sc-transfected cells). The ratio of luciferase activity was calculated either in the presence or absence of miR-210. C, immunofluorescence staining of MSCs stained for FLASH/Casp8ap2 (red; indicated by white arrows) and its overlay images of nuclei stained with DAPI (blue). Compared with its nuclear localization under normoxia, anoxia translocated FLASH/CASP8AP2 from the nucleus into the cytoplasm as indicated by lack of red fluorescence in the nuclei. The percentage of cells (at ×40) with FLASH/CASP8AP2-positive nuclei was significantly higher in the cells cultured under normoxia (p < 0.01 versus cells treated with anoxia for 6 h). D, RT-PCR showing the effect of preconditioning on Casp8ap2 gene expression in MSCs under 6 h of anoxia. Casp8ap2 gene expression was significantly abolished in MSCs preconditioned by two 30-min cycles of I/R compared with non-PCMSCs and MSCs preconditioned by 1 cycle of I/R (* versus other groups, p < 0.01).