Abstract
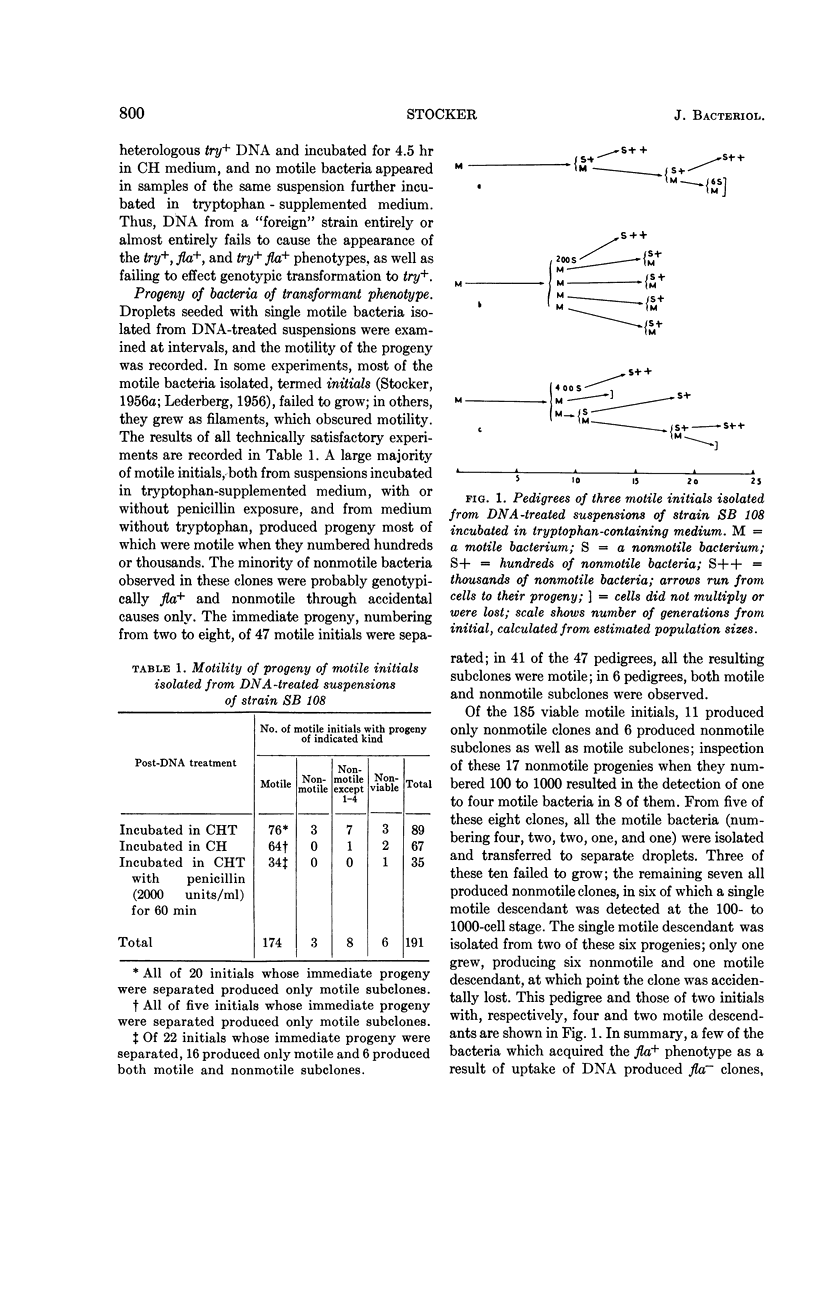
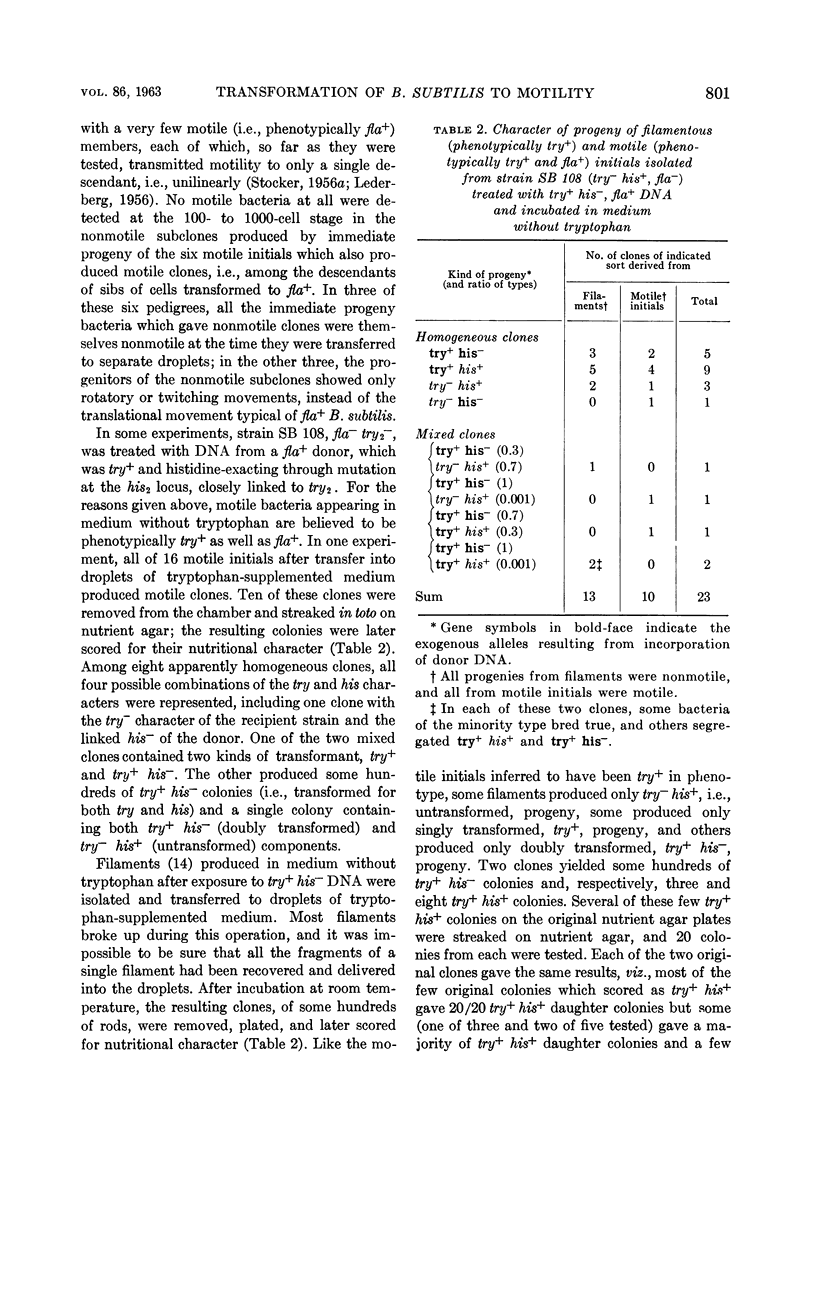
Stocker, B. A. D. (Stanford Medical Center, Palo Alto, Calif.). Transformation of Bacillus subtilis to motility and prototrophy: micromanipulative isolation of bacteria of transformed phenotype. J. Bacteriol. 86:797–804. 1963.—A nonmotile (nonflagellated, fla−) try− strain of Bacillus subtilis was transformed to fla+ and to try+ by wild - type deoxyribonucleic acid (DNA) at comparable rates. Bacteria of fla+ phenotype were recognized by their motility approximately 3 hr after uptake of DNA, and bacteria of try+ phenotype at about the same time by their elongation into filaments in a medium lacking tryptophan. Of phenotypically transformed bacteria of each sort isolated by micromanipulation, the majority produced only transformed progeny, a mixture of transformed and untransformed, or a mixture of two kinds of transformant. Some produced only untransformed progeny, or progeny transformed only at a locus linked to that concerned in their phenotypic transformation. In a few clones, some partial heterozygotes were present even ten generations after DNA uptake. In nonmotile clones derived from motile isolates, the unilinear transmission of motility to one to four descendants was detected; it is attributed to persistence of a corresponding number of units of some product of an unincorporated fla+ gene, probably flagella or cell walls each carrying several flagella. No pedigrees indicating unilinear transmission of an unincorporated fla+ gene were observed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LACKS S., HOTCHKISS R. D. Formation of amylomaltase after genetic transformation of pneumococcus. Biochim Biophys Acta. 1960 Dec 4;45:155–163. doi: 10.1016/0006-3002(60)91436-0. [DOI] [PubMed] [Google Scholar]
- LEIFSON E. Staining, shape and arrangement of bacterial flagella. J Bacteriol. 1951 Oct;62(4):377–389. doi: 10.1128/jb.62.4.377-389.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Linear Inheritance in Transductional Clones. Genetics. 1956 Nov;41(6):845–871. doi: 10.1093/genetics/41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., STOCKER B. A. BIOSYNTHETIC LATENCY IN EARLY STAGES OF DEOXYRIBONUCLEIC ACIDTRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1963 Oct;86:785–796. doi: 10.1128/jb.86.4.785-796.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. An environmentally-induced transition from the flagellated to the non-flagellated state in Salmonella typhimurium: the fate of parental flagella at cell division. J Gen Microbiol. 1962 Jun;28:257–270. doi: 10.1099/00221287-28-2-257. [DOI] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. The occurrence of rare motile bacteria in some non-motile Salmonella strains. J Gen Microbiol. 1957 Oct;17(2):424–436. doi: 10.1099/00221287-17-2-424. [DOI] [PubMed] [Google Scholar]
- QUADLING C. The unilinear transmission of motility and its material basis in Salmonella. J Gen Microbiol. 1958 Feb;18(1):227–237. doi: 10.1099/00221287-18-1-227. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Abortive transduction of motility in Salmonella; a nonreplicated gene transmitted through many generations to a single descendant. J Gen Microbiol. 1956 Dec;15(3):575–598. doi: 10.1099/00221287-15-3-575. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]


