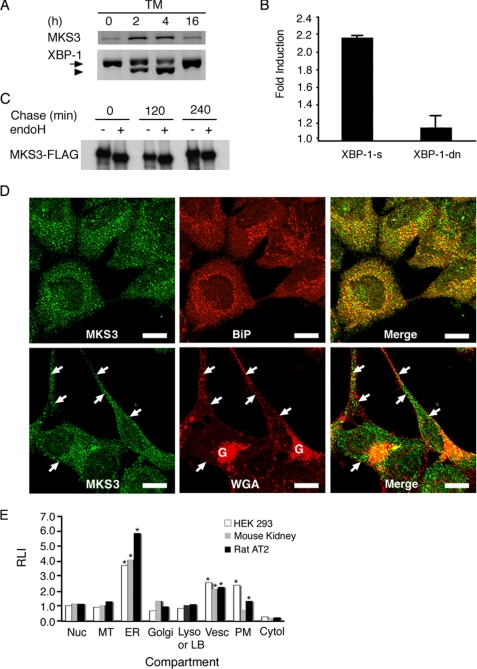
FIGURE 1.
MKS3 is an ER stress response gene. A, HEK293 cells were treated with tunicamycin (TM) for the indicated number of hours. Reverse transcription-PCR was performed for MKS3 and XBP-1. The arrow indicates unspliced XBP-1, and the arrowhead indicates spliced (active) XBP-1. B, HEK293 cells were transiently transfected with an MKS3 promoter (−97 to −1064)-luciferase construct, pRL-TK vector encoding Renilla luciferase, and plasmid encoding spliced (XBP-1-s) or dominant-negative (XBP-1-dn) forms of XBP-1. Firefly luciferase activity was normalized to Renilla luciferase activity. Results are expressed as -fold induction of luciferase activity relative to vector-transfected cells and represent the average of three independent experiments. C, HEK293 cells were transfected with MKS3-FLAG. 24 h later, cells were labeled with [35S]Met/Cys for 30 min, after which the labeling medium was replaced with chase medium. Cell lysates were immunoprecipitated with anti-FLAG antibody, treated with endoglycosidase H (endoH), and analyzed by SDS-PAGE/autoradiography. D, endogenous MKS3 in HEK293 cells was detected by confocal microscopy using antibody directed against recombinant human MKS3 (see supplemental Fig. S1D). Anti-BiP antibody was used as a marker for ER (upper panels), whereas WGA was used to stain the plasma membrane and Golgi apparatus (lower panels). The arrows indicate colocalization of MKS3 and WGA at the plasma membrane. Scale bars = 10 μm. E, HEK293 cells, rat lung, and mouse kidney were prepared for cryoultramicrotomy and immunogold labeling with anti-MKS3 antibody/protein A-gold. Subcellular localization of MKS3 was assessed by quantitation of gold particles and is expressed as the relative labeling index (RLI). Cytol, cytosol; LB, lamellar body; Lyso, lysosome; MT, mitochondria; Nuc, nucleus; PM, plasma membrane; vesc, vesicle. The asterisks identify subcellular compartments with significant accumulation of MKS3 as defined under “Experimental Procedures.”