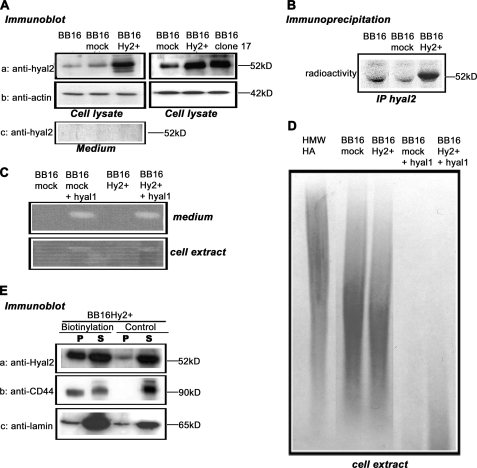
FIGURE 1.
Quantification of Hyal2 expression and its hyaluronidase activity. v-src-transformed rat fibroblasts (BB16) were stably transfected with either an empty plasmid (BB16mock clone) or a plasmid expressing Hyal2 (BB16Hy2+ clone and clone 17). A, Western blot analyses of cell lysates (5 μg of proteins) and concentrated media with anti-Hyal2 (P16) and anti-actin antibodies. B, immunoprecipitation of Hyal2 using P16 in cells labeled with [35S]methionine for 18 h. C, zymographic measurements of hyaluronidase activity at pH 3.7 in BB16mock and BB16Hy2+ cells, and in the same cells transiently transfected with rat Hyal1 cDNA (+Hyal1). D, measurements of HA-degrading activity in BB16mock and BB16Hy2+ cells, and in the same cells transiently transfected with rat Hyal1 cDNA (+Hyal1). Cell extracts were incubated with 2.5 × 106 Da HA for 16 h at pH 3.7, followed by agarose gel electrophoresis of the resulting incubate and detection of HA with Stains-All, allowing assessment of HA amount and size. The first lane is the high molecular mass HA incubated for 16 h at pH 3.7 with the buffer only. E, Western blots of BB16Hy2+ cells using anti-Hyal2 (P16) and anti-CD44 (OX49) antibodies following cell surface biotinylation and precipitation of the biotinylated proteins with streptavidin-Sepharose beads. The signal in the pellet (P) represents half the amount of Hyal2, CD44, or lamin present on the cell surface. The signal in the supernatant (S) represents 1/10th the amount of these proteins that was inaccessible to external biotin. A control experiment without biotinylation is shown. Anti-lamin antibodies were used to detect a typical nuclear protein.