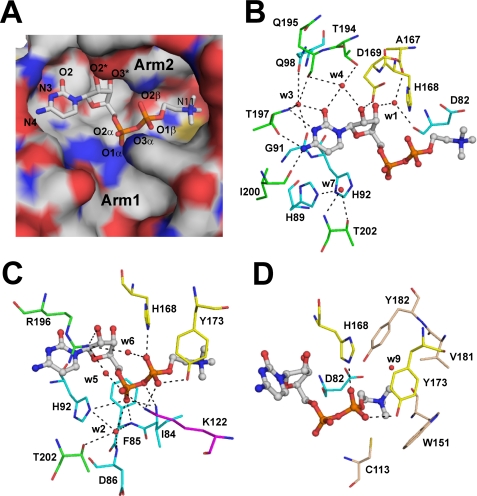
FIGURE 5.
CDP-choline in the active site of the CCT236. A, electrostatic surface representation of the active site pocket. Arg-196 and Tyr-173 were omitted from the surface representation to avoid obscuring the view of bound product. Arg-196 and Tyr-173 side chains extend over the cytidine base and trimethylammonium group, respectively (see C). B, interaction of cytidine in the active site. The hydrogen bonding interactions with the residues in the conserved RTEGISTS motif (green), HXGH motif/L1 loop (cyan), L5 loop (yellow), and solvent molecules are shown by dashed lines. The water molecules are labeled by the last digit of their ID numbers in the PDB entry. C, interaction of the phosphate groups in the active site. Lys-122 from loop L2 is shown in magenta; other elements are color-coded as in B. D, residues surrounding the choline group. Aromatic side chains as well as electrostatic interaction with Asp-82 stabilize the positive charge on the trimethylammonium group.