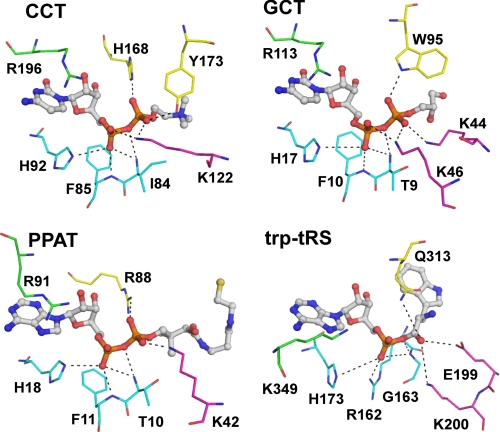
FIGURE 7.
Comparison of active site residues between nucleotidylyltransferases. CCT, residues shown to directly coordinate the α- and β-phosphates of the bound product in CCT236 are shown with CDP-choline in stick format. Residues belonging to loop L1 (conserved FDLFHXGH motif) is colored in cyan, Lys-122 from loop L2 in magenta, and both His-168 and Tyr-173 from loop L5 in yellow. Arg-196 from loop L6 (conserved RTEGISTS) that contacts the α-phosphate via a water molecule is also shown in green. Analogous residues present in other nucleotidyltransferases are shown with the same color coding schemes. GCT, glycerol-phosphate cytidylyltransferase with CDP-glycerol (PDB code 1N1D); PPAT, phosphopantetheine adenylyltransferase with dephospho-coenzyme A (PDB code 1B6T); Trp-tRS, human tryptophanyl-tRNA synthetase with Trp-AMP (PDB code 2QUJ). Note the similar ligand conformations in all four active sites.