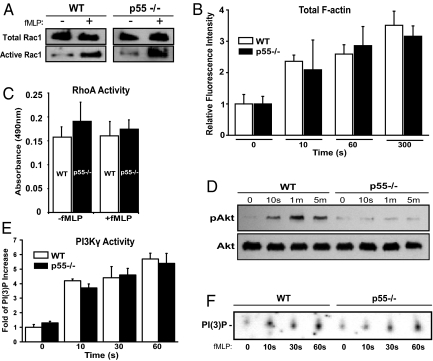
Fig. 3.
Biochemical characterization of p55−/− neutrophils. (A) Rac1 activation. Neutrophils were stimulated with 1 μM of fMLP and lysate was analyzed by the pull-down assay. Western blotting was performed using an anti-Rac1 monoclonal antibody. No difference was observed in the total or active Rac1 between WT and p55−/− neutrophils. Blot is representative of three separate experiments. (B) Total F-actin polymerization. Neutrophils were stimulated with 1 μM fMLP, fixed in cold paraformaldehyde, permeabilized, and incubated for 1 h with 0.2 μM FITC-conjugated Phalloidin. Cells were washed and analyzed by flow cytometry. Bars represent the mean fluorescence intensity relative to time 0 (no fMLP). No difference was observed in the polymerized F-actin between WT and p55−/− neutrophils (graph represents three experiments). (C) RhoA activation. RhoA activity was measured using an ELISA-based kit (Cytoskeleton Inc). RhoA activation was comparable between WT and p55−/− neutrophils (graph represents three experiments). (D) Phosphorylation of Akt. WT and p55−/− neutrophils were stimulated with 1 μM fMLP and lysates were analyzed by Western blotting using a polyclonal antibody against Akt. This antibody detects phosphorylated threonine-308. Total Akt blotting was used to confirm equal loading in each lane. Blot is representative of four separate experiments. (E) Activity of PI3Kγ. PI3Kγ was immunoprecipitated from WT and p55−/− neutrophils, and its activity was measured by in vitro production of PI(3)P. The intensity of the PI(3)P spots were normalized to time 0, and the data shown represents three separate experiments. (F) A representative autoradiograph of one PI3Kγ activity assay.