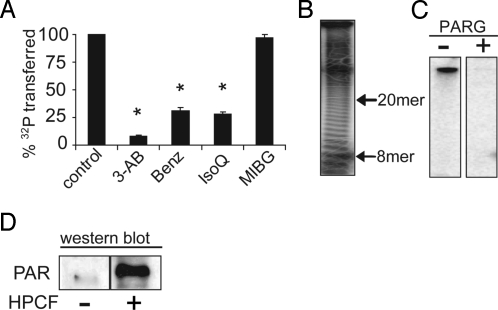
Fig. 4.
PARP-1 is activated in an HPCF-dependent manner to catalyze self-modification with PAR. PARP-1 (50 pM) along with either [32P-adenylate] NAD (50 μM) (A–C) or non-radiolabeled NAD (D) were incubated at 25 °C in the absence or presence of HPCF (50 μg/mL). (A) The reactions were carried out in the absence (control) or presence of 3-aminobenzamide (3-AB, 3 mM), benzamide (Benz, 3 μM), isoquinolinone (IsoQ, 1 μM), or m-iodobenzylguanidine (MIBG, 100 μM). * indicates that differences in % 32P transferred relative to the control lacking inhibitor are statistically significant (P < 0.05). (B) The completed reactions were incubated at 60 °C in the absence or presence of NaOH (100 mM). After 60 min, released PAR was resolved by high percentage TBE acrylamide gels (19% acrylamide, 1% bis-acrylamide), and visualized after autoradiography. The 8-mer and 20-mer PAR chain, which co-migrate with the bromophenol blue and xylene cyanol loading dyes respectively (35, 36) are indicated next to the autoradiographs. (C) The reactions were further incubated at 25 °C in the absence or presence of PARG (100 ng/mL). After 15 min, the samples were fractionated by SDS/PAGE, and scored for 32P-incorporation by SDS/PAGE followed by autoradiography of the dried gels. (D) After 15 min, the samples were probed for PAR by Western blot analysis using anti-PAR antibodies.