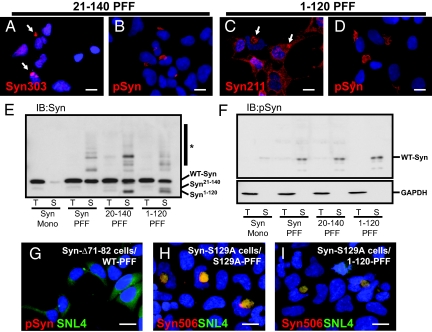
Fig. 4.
Inclusion formation does not require the α-Syn N- or C-terminal regions in seeds. (A–D) QBI-A53T-Syn cells were transduced with α-Syn PFFs lacking either the N-terminal (α-Syn21–140) or C-terminal domain (α-Syn1–120). Transduced cells were immunostained using antibodies recognizing either the extreme N-terminal (Syn303; A) or C-terminal (Syn211; C); thus, detecting only endogenous α-Syn. Both truncated forms of fibrils recruited cellular α-Syn as indicated by inclusion formation. Inclusions were phosphorylated, indicating that their formation does not depend on seed phosphorylation or interaction with membranes. (E and F) Lysates from cells transduced with either WT-α-Syn monomer (Mono), full-length (Syn PFF), or truncated α-Syn fibrils were probed with antibodies against α-Syn (SNL4) and α-SynpSer129. Immunoblot with SNL-4 (E) shows full-length endogenous α-Syn within Triton-insoluble fractions of cells transduced with WT, α-Syn21–140, and α-Syn1–120 PFFs, indicating that cellular α-Syn is converted by fibril seeds. Transduction also resulted in the appearance of high molecular weight α-Syn species (*) consistent with ubiquitination. Fibril transduction also led to a dramatic increase in the amount of phosphorylated α-Syn found almost exclusively within SDS-soluble fractions (F Upper) consistent with its location within insoluble inclusions. GAPDH loading controls are also shown (F Lower). (G) QBI-Δ71-82-Syn cells transduced with WT fibrils did not form inclusions, indicating that the core fibril assembly region of α-Syn is critical to recruitment and incorporation. (H and I) QBI-cells stably expressing α-Syn mutated at Ser-129 (S129A) were transduced with PFFs prepared from α-Syn-S129A (H) or α-Syn1–120 (I), which lack this phosphorylation site. Double immunostaining with Syn506 and SNL4 indicate the formation of misfolded α-Syn inclusions. [Scale bars, 5 μm (A–D); 5 μm (G–I).]