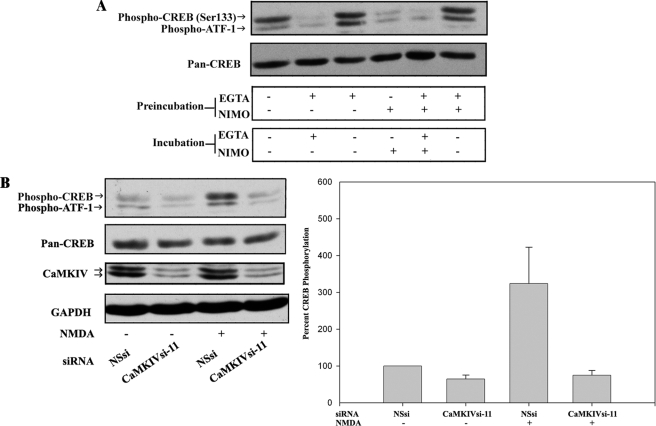
FIGURE 2.
CaMKIV-dependent CREB/ATF-1 phosphorylation is downstream of VDCC and NMDA receptor activities. A, removal of extracellular Ca2+ and inhibition of VDCC activity reversibly inhibit CREB/ATF-1 phosphorylation. BE(2)C cells were serum-starved for 3 h and then preincubated for 15 min in medium (containing ∼1 mm calcium) without additions or in the same medium with the addition of either 2 mm EGTA, 10 μm nimodipine (NIMO), or 2 mm EGTA plus 10 μm nimodipine. Media were replaced as indicated, and cells were incubated for an additional 15 min and then analyzed by immunoblotting for CREB/ATF-1 phosphorylation and total CREB (Pan-CREB) expression. B, NMDA-induced CREB/ATF-1 phosphorylation is blocked by RNAi of CaMKIV. BE(2)C cells were transfected with 50 nm NSsi or CaMKIVsi-11 siRNAs for 48 h, serum-starved for 3 h, and then preincubated with HEPES-Tyrode's buffer with glycine substituted for Mg2+ (119 mm NaCl, 2.5 mm CaCl2, 2 mm glycine, 25 mm HEPES, 30 mm glucose) for 15 min. Cultures were then treated with 7.5 μm NMDA or vehicle control (DMSO; −) for 2 min in the same buffer and then analyzed by immunoblotting for CREB/ATF1 phosphorylation and total CREB expression. Blots were also probed for CaMKIV and GAPDH to confirm silencing of CaMKIV and equivalent protein loading, respectively. Right, CREB phosphorylation was quantified by scanning densitometry of phospho-CREB immunoreactivity normalized to total CREB (Pan-CREB) immunoreactivity and expressed as a percentage of normalized CREB phosphorylation in vehicle-treated/NSsi-transfected cells.