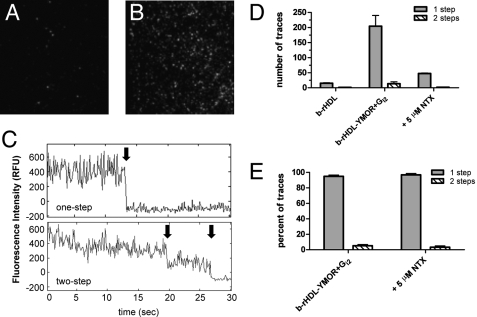
FIGURE 5.
Single-molecule imaging of Cy3-labeled agonist binding to rHDL·YMOR + Gi2 confirms that the majority of YMOR is monomeric when reconstituted into HDL. Binding of [Lys7, Cys8]dermorphin-Cy3, a fluorescently labeled MOR-specific agonist, to rHDL·YMOR+Gi2 was observed with prism-based total internal reflection fluorescence microscopy. YMOR was reconstituted into HDL particles using biotinylated apoA-1, followed by Gi2 heterotrimer addition at a 30:1 G protein to receptor molar ratio. Reconstituted HDL (A) or rHDL·YMOR+Gi2 (B) were then incubated with a saturating concentration (500 nm) of [Lys7, Cys8]dermorphin-Cy3, adhered to a streptavidin-coated quartz slide, and washed with ice-cold 25 mm Tris·HCl, pH 7.7 buffer. Bound [Lys7, Cys8]dermorphin-Cy3 was continuously excited at 532 nm to observe photobleaching of the fluorophore. C, representative fluorescence intensity traces for a one- and two-step photobleach event are shown. The arrows indicate photobleach events. D and E, quantification of photobleaching showed that ∼95% of the bound [Lys7, Cys8]dermorphin-Cy3 bleached in a single step, suggesting that 95% of rHDL particles contained monomeric YMOR. [Lys7, Cys8]dermorphin-Cy3 binding was reversible, as shown the addition of 5 μm NTX. A minimum of four slide regions and 200 fluorescent spots were counted for each sample.