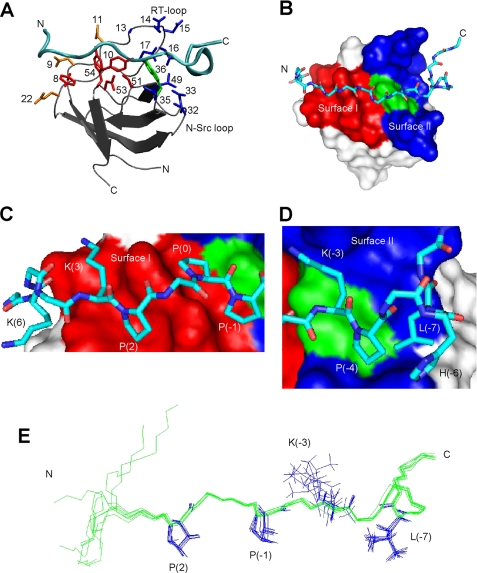
FIGURE 1.
The structure of the AbpSH3·ArkA peptide complex. A, schematic of the AbpSH3·ArkA complex. The SH3 domain is in silver with the peptide in cyan. Side chains of conserved surface I residues within yeast SH3 domains (Y8, Y10, P51, N53, Y54) are shown in red, key surface II residues for AbpSH3 (Ala13, Glu14, Asp15, Asn16, Glu17, Val32, Asp33, Asp35, and Leu49) are shown in blue, Trp36, which is at the border of surfaces I and II is shown in green. Asp9, Asp11, and Glu22, which interact with the N terminus of the peptide, are in orange. B, surface representation of the AbpSH3 highlighting surface I (red) and surface II (blue). The surface of Trp36 is indicated in green. The ArkA peptide is represented in stick, with nitrogen in dark blue, carbon in light blue, and oxygen in red. C, interactions of the PPII-helical region of ArkA with binding surface I. The positions of key peptide side chains are indicated. D, interactions of the extended region of the ArkA peptide with surface II. E, overlay of eight low energy ArkA peptide conformers.