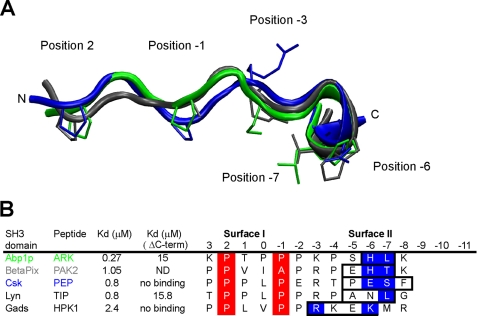
FIGURE 6.
Similarity of the ArkA peptide conformation with other extended peptides bound to SH3 domains. A, structural alignment of the central 12 residues from of bound ArkA (green, this study), with bound PAK2 (gray, root mean square deviation = 1.4 Å, pdb: 2DF6) and PEP (blue, root mean square deviation = 1.9 Å, pdb: 1JEG). The side chains at key positions, 2, −1, −3, −6, and −7 are shown. B, alignment of the peptide sequences shown in A, including two extra peptides found in other complexes. The helical element is boxed, the surface I PXXP motif is highlighted in red, and the key surface II contact residues within the helical element are highlighted in blue. Kd values are shown for the WT peptides and peptides where the boxed residues are mutated or deleted (ΔC-term). ND, not determined.