To the Editor:
Beare-Stevenson syndrome (BSS; OMIM 123790) is a rare craniosynostosis syndrome characterized by cutis gyrata, acanthosis nigricans, skin tags, anogenital anomalies and a prominent umbilicus. Two activating point mutations in FGFR2, p.Y375C and p.S372C, account for 50–60% of patients with BSS, and locus and/or allelic heterogeneity have been proposed. We present a novel deletion of 21 amino acids in the FGFR2 gene, c.859del63, in a patient with BSS. BSS results from a gain of FGFR2 function, and this deletion may act by altering the splicing of isoform IIIc, resulting in illegitimate expression and thus a gain of function of FGFR2b.
The propositus was delivered by cesarean for a dizygotic twin pregnancy at term to a 40-year-old, G3 P2-4 mother and a 48-year-old father. Birth parameters were weight 3,860 g (75–90th centile for a singleton male), length 51 cm (50–75th centile for a singleton male) and head circumference 36.5 cm (75–90th centile for a singleton male). Striking craniofacial features were present, including ocular proptosis with hypoplasia of the supraorbital ridges, hypertelorism with a divergent strabismus, deep creases below his eyes, a high nasal bridge, midface hypoplasia, complete atresia of the external ear canals with a left preauricular pit and bilateral ear creases. There were multiple neonatal teeth, gingival hyperplasia and a high-arched, narrow palate with a bifid uvula. Cutaneous features included cutis gyrata and acanthosis nigricans of the posterior scalp, small skin tags at the corners of the mouth, excess neck skin and a prominent umbilicus with redundant skin. A sacral tag, hypospadias and a prominent scrotal raphé were present. Examination of the hands and feet showed redundant palmar and plantar skin, slightly broad thumbs and halluces and mild skin syndactyly of the second and third toes with overlapping fourth toes. Investigations for a flat occiput and a prominent forehead at four months of age showed fusion of the posterior aspect of the sagittal and the proximal lambdoid sutures and cranial vault remodeling was performed at seven months. Bilateral choanal stenosis and a mild to moderate mixed hearing loss requiring hearing aids were present. Ophthalmological examination detected mild optic nerve hypoplasia and high myopia with astigmatism requiring corrective lenses. Obstructive sleep apnea was treated with a tracheostomy and a ventriculoperitoneal shunt was placed because of an Arnold-Chiari malformation with hydrocephalus. His length was 107 cm at age six years (3rd centile), but his other growth parameters were normal. He has had initial mild motor delays, but the propositus’ neurodevelopmental progress was normal.
A clinical diagnosis of BSS was made shortly after birth. At that time, targeted re-sequencing for the two previously known FGFR2 mutations, p.Y375 C and p.S372 C [Przylepa et al., 1996], was performed on DNA extracted from peripheral blood lymphocytes from the propositus, and was negative.
The diagnosis of a rhabdomyosarcoma in his twin when both were five years of age prompted further re-sequencing of FGFR2 in the propositus, and a de novo, intragenic deletion of 63 nucleotides starting in exon 8 of the gene, c.859del63, was detected (Fig. 2). This deletion predicts the loss of 21 amino acids from the FGFR2 protein. This mutation was considered to be pathogenic because of the classical BSS phenotype in the propositus and the location of the deletion in FGFR2. Table 1 summarizes the clinical features of BSS patients with known FGFR2 mutations, including this child. Both parents and his dizygotic twin brother were sequenced for the same mutation, and all were negative (data not shown). Neither his parents or twin brother showed any signs of BSS, and both parents have had normal health.
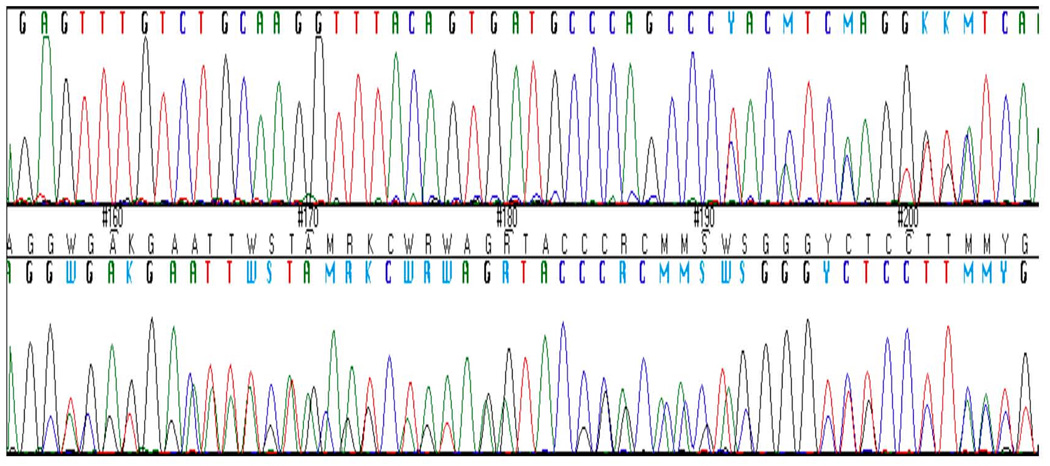
Fig. 2.
Forward chromatogram showing the start of the deletion, c.859del63 (indicated by arrow) in FGFR2 in the propositus. Numbering is from first nucleotide of start codon (A) = 1. The deletion is exonic and results in the loss of amino acids 287–308 of the protein.
Table I.
Beare-Stevenson Syndrome Clinical Features Compared to the Propositus in this Report
| Clinical Feature | p.Y375C (n = 9) | p.S372C (n = 2) | This patient |
|---|---|---|---|
| Craniofacial Anomalies | |||
| Cloverleaf skull/fused sutures | 9/9 | 1/2 | + |
| Proptosis | 9/9 | 2/2 | + |
| Hypertelorism | 7/9 | 2/2 | + |
| Midface hypoplasiaa | 5/9 | 2/2 | + |
| Choanal atresia/stenosis | 6/9 | 1/2 | + |
| Brain and Eye Anomalies | |||
| Arnold Chiari malformationb | 5/9 | 1/2 | + |
| Optic hypoplasia/atrophy | 0/9 | 1/2 | + |
| Palate/Dentition | |||
| Orofacial clefting | 1/9 (uvula) | 0/2 | + |
| High palate | 3/9 | 1/2 | + |
| Natal teeth | 0/9 | 0/2 | + |
| Partial anodontia | 0/9 | 0/2 | + |
| Dermatological Anomalies | |||
| Cutis gyrata | 9/9 | 2/2 | + |
| Skin furrows – preauricular | 7/9 | 1/2 | + |
| Skin furrows – hands and feet | 5/9 | 1/2 | + |
| Acanthosis nigricans | 3/9 | 1/2 | + |
| Skin tags | 5/9 | 1/2 | + |
| Nail hypoplasia | 1/9 | 2/2 | + |
| Gastrointestinal System | |||
| Prominent umbilicus | 8/9 | 2/2 | + |
| Anterior/imperforate anus | 5/9 | 1/2 | + |
| Sacral appendage/sacral anomaly | 3/9 | 0/2 | + |
| Genitourinary Tract | |||
| Bifid scrotum | 1/3 | 1/1 | − |
| Hypospadias | 1/3 | 0/1 | + |
| Hypoplastic labia minora | 1/5 | 1/1 | NA |
| Prognosis | |||
| Tracheostomy | 2/9 | 2/2 | + |
| Ventriculoperitoneal shunt | 3/9 | 1/2 | + |
| Survival | |||
| Death prior to 2 years | 4/7 | 2/2 | No |
Included cases were: p.S372C: [Hall et al., 1992, patient 1]; [Fonseca et al., 2008].
p.Y375C: [Hall et al., 1992, patient 4]; [Przylepa et al., 1996]; [Krepelová et al., 1998]; [Wang et al., 2002]; [Akai et al., 2002]; [Vargas et al., 2003, patients 1 and 2]; [McGaughran et al., 2006]; [Eun et al., 2007].
Midface hypoplasia - includes downslanting palpebral fissures;
Arnold-Chiari malformation – includes hydrocephalus;
NA = not applicable.
The four fibroblast growth factor receptors (FGFRs) share similar organization, with an extracellular domain that has three immunoglobulin-like (Ig-like) domains (IgI, IgII, IgIII), a transmembrane region, and a cytoplasmic region containing a catalytic protein tyrosine kinase core and additional regulatory sequences. The Ig-like domains bind fibroblast growth factors (FGFs) resulting in the dimerization of adjacent FGFRs and phosphorylation and activation of the tyrosine kinase domain [Bochukova et al., 2008]. Three exons of FGFR2 (IgIIIa, b, and c) may be alternatively spliced to form either FGFR2b (IgIIIa and IgIIIb), which is expressed in ectoderm and binds FGF1, FGF3, FGF7 and FGF10, or FGFR2c (IgIIIa and IgIIIC), which is expressed in mesenchyme and does not bind FGF10 [Bochukova et al., 2008]. The deletion present in the propositus was located in exon 8 of FGFR2 that encodes the IgIIIa domain. Mutations in IgIIIa have previously been associated with Crouzon syndrome [OMIM123500], Pfeiffer syndrome [OMIM 101600] and Jackson-Weiss syndrome [OMIM123150]. In Crouzon syndrome, the mutations commonly create or destroy a cysteine residue, resulting in an unpaired cysteine that forms a disulphide bond and leads to constitutive activation of FGFR2 (gain of function) independent of ligand binding [Meyers et al., 1996; Lajeunie et al., 2006]. Similarly, the two previously identified BSS mutations, p.Y375C and p.S372C, have been hypothesized to affect both the BEK (FGFR2c) and KGFR (FGFR2b) isoforms of FGFR2 and cause disease by constitutive activation of these isoforms, although functional studies have not been performed [Przylepa et al., 1996]. Deletions of FGFR2 are exceedingly rare - a frameshift deletion of IIIc has been described (958_959delAC, causing p.T320GfsX5 in a family with Jackson-Weiss syndrome [Lajeunie et al., 2006]) and a 1.93 kb deletion removing IIIc has been described in Apert syndrome [Bochukova et al., 2008]. These deletions are thought to cause disease by altering the splicing of isoform IIIc, resulting in illegitimate expression (and thus a gain of function) of FGFR2b in fibroblasts, which are mesenchymally derived, and normally only express FGFR2c. A similar activating mechanism, although unproven, could be present in this child.
In summary, we present a novel FGFR2 deletion, c.859del63, in a male with some typical features of BSS, including a craniofacial appearance similar to Crouzon syndrome and additional dermatological findings of cutis gyrata, acanthosis nigricans, skin furrows with loose palmar and plantar skin, skin tags and a prominent umbilicus. His atypical findings for BSS, for example, neonatal teeth and external auditory canal atresia, add to the phenotypic information for this condition.
Fig. 1.
Face of the propositus at 6 years of age.
ACKNOWLEDGEMENTS AND FUNDING
Anne Slavotinek was generously funded by a K08 grant, K08HD053476-01A1, from the National Institute of Child Health and Development (NICHD), National Institutes of Health (NIH).
REFERENCES
- Akai T, Iizuka H, Kishibe M, Kawakami S, Kobayashi A, Ozawa T. A case of Beare-Stevenson cutis gyrata syndrome confirmed by mutation analysis of the fibroblast growth factor receptor 2 gene. Pediatr Neurosurg. 2002;37:97–99. doi: 10.1159/000065112. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Roscioli T, Hedges DJ, Taylor IB, Johnson D, David DJ, Deininger PL, Wilkie AO. Rare mutations of FGFR2 causing apert syndrome: identification of the first partial gene deletion, and an Alu element insertion from a new subfamily. Hum Mutat. 2008;30:204–211. doi: 10.1002/humu.20825. [DOI] [PubMed] [Google Scholar]
- Eun SH, Ha KS, Je BK, Lee ES, Choi BM, Lee JH, Eun BL, Yoo KH. The first Korean case of Beare-Stevenson syndrome with a Tyr375Cys mutation in the fibroblast growth factor receptor 2 gene. J Korean Med Sci. 2007;22:352–356. doi: 10.3346/jkms.2007.22.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Costa-Lima MA, Cosentino V, Orioli IM. Second case of Beare-Stevenson syndrome with an FGFR2 Ser372Cys mutation. Am J Med Genet A. 2008;146A:658–660. doi: 10.1002/ajmg.a.32176. [DOI] [PubMed] [Google Scholar]
- Hall BD, Cadle RG, Golabi M, Morris CA, Cohen MM., Jr Beare-Stevenson cutis gyrata syndrome. Am J Med Genet. 1992;44:82–89. doi: 10.1002/ajmg.1320440120. [DOI] [PubMed] [Google Scholar]
- Krepelová A, Baxová A, Calda P, Plavka R, Kapras J. FGFR2 gene mutation (Tyr375Cys) in a new case of Beare-Stevenson syndrome. Am J Med Genet. 1998;76:362–364. [PubMed] [Google Scholar]
- Lajeunie E, Heuertz S, El Ghouzzi V, Martinovic J, Renier D, Le Merrer M, Bonaventure J. Mutation screening in patients with syndromic craniosynostoses indicates that a limited number of recurrent FGFR2 mutations accounts for severe forms of Pfeiffer syndrome. Eur J Hum Genet. 2006;14:289–298. doi: 10.1038/sj.ejhg.5201558. [DOI] [PubMed] [Google Scholar]
- McGaughran J, Sinnott S, Susman R, Buckley MF, Elakis G, Cox T, Roscioli T. A case of Beare-Stevenson syndrome with a broad spectrum of features and a review of the FGFR2 Y375C mutation phenotype. Clin Dysmorphol. 2006;15:89–93. doi: 10.1097/01.mcd.0000194407.92676.9d. [DOI] [PubMed] [Google Scholar]
- Meyers GA, Day D, Goldberg R, Daentl DL, Przylepa KA, Abrams LJ, Graham JM, Jr, Feingold M, Moeschler JB, Rawnsley E, Scott AF, Jabs EW. FGFR2 exon IIIa and IIIc mutations in Crouzon, Jackson-Weiss, and Pfeiffer syndromes: evidence for missense changes, insertions, and a deletion due to alternative RNA splicing. Am J Hum Genet. 1996;58:491–498. [PMC free article] [PubMed] [Google Scholar]
- Przylepa KA, Paznekas W, Zhang M, Golabi M, Bias W, Bamshad MJ, Carey JC, Hall BD, Stevenson R, Orlow S, Cohen MM, Jr, Jabs EW. Fibroblast growth factor receptor 2 mutations in Beare-Stevenson cutis gyrata syndrome. Nat Genet. 1996;13:492–494. doi: 10.1038/ng0896-492. [DOI] [PubMed] [Google Scholar]
- Vargas RA, Maegawa GH, Taucher SC, Leite JC, Sanz P, Cifuentes J, Parra M, Munoz H, Maranduba CM, Passos-Bueno MR. Beare-Stevenson syndrome: Two South American patients with FGFR2 analysis. Am J Med Genet A. 2003;121A:41–46. doi: 10.1002/ajmg.a.20101. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Huang CB, Tsai FJ, Wu JY, Lai RB, Hsiao M. Mutation in the FGFR2 gene in a Taiwanese patient with Beare-Stevenson cutis gyrata syndrome. Clin Genet. 2002;61:218–221. doi: 10.1034/j.1399-0004.2002.610309.x. [DOI] [PubMed] [Google Scholar]