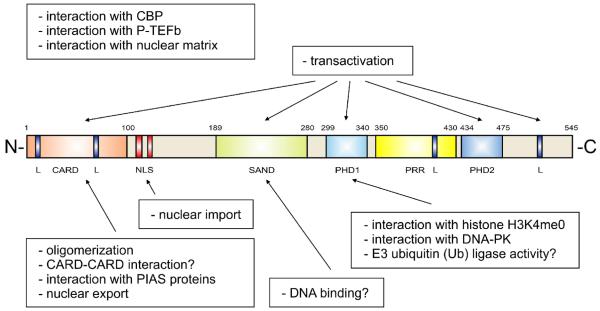
Figure 1. Schematic representation of human AIRE protein.
The domains and functional elements of the AIRE protein are shown in different colors; CARD (caspase-recruitment domain), SAND (Sp100, AIRE-1, NucP41/P75 and Drosophila DEAF-1), PHD (plant homeodomain), PRR (proline-rich region), L (LXXLL motive), NLS (nuclear-localization signal). Functions of AIRE are shown in boxes and indicated by arrows pointing to the corresponding domain, if known. The CARD (caspase-recruitment domain) has been implicated in homo-oligomerization and is required for nuclear-dot formation and for the heterodimerization of CARD proteins that function in inflammation or apoptosis. Interaction of the CARD with PIAS1 (Protein Inhibitors of Activated STAT) proteins influences the transactivation ability of AIRE. SAND is a putative DNA-binding domain. The first PHD finger has been shown to interact with histone H3 unmethylated at lysine 4 and DNA-PK (DNA protein kinase). Both of these interactions are required for proper transactivation. In addition, PHD1 has been shown to have E3 ubiquitin ligase activity, a mechanism by which a ubiquitin molecule is covalently attached to the target protein. AIRE has also been shown to interact with the transcriptional coactivator CBP (CREB binding protein) and positive transcription elongation factor b (P-TEFb). The latter interaction indicates that AIRE might function in elongation rather than the initiation of transcription. In addition, AIRE has shown to bind the nuclear matrix.