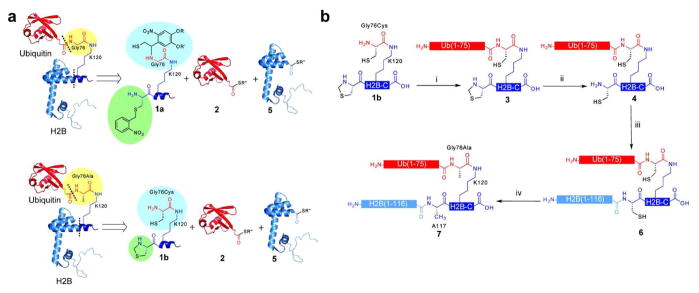
Figure 1. Semisynthesis of uH2BG76A.
a, Retrosynthetic comparison of uH2B (top) and uH2BG76A (bottom) syntheses. Both were generated via a 3-piece ligation strategy with the following polypeptides: synthetic peptide containing residues 117–125 of H2B and bearing an A117C mutation, H2B-C, 1a and 1b; recombinant ubiquitin(1-75)-α-thioester 2; and recombinant H2B(1-116)-α-thioester 5. For the semisynthesis of uH2BG76A, the ligation auxiliary was replaced with a cysteine (blue) and the photolytically removable cysteine protecting group was replaced with a thiazolidine (green). The resultant ubiquitylated proteins differ only at position 76 of ubiquitin (yellow). Dashed lines indicate junctions formed by EPL reactions. b, Synthetic scheme for the generation of uH2BG76A. i) EPL was used to ligate peptide 1b to protein 2, forming branched protein 3. ii) Ligation product 3 was treated with methoxylamine at pH 5, affording 4. iii) Ligation of protein 4 to protein 5, forming uH2BA117C/G76A, 6. iv) Raney nickel or radical-initiated desulfurization of protein 6, forming uH2BG76A, 7. R = CH2CH2CH2C(O)NHCH3; R′ = CH3; R″ = CH2CH2SO3H.