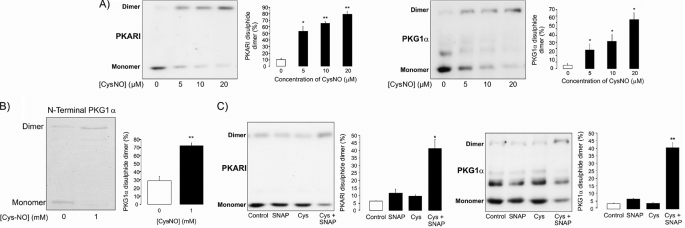
FIGURE 2.
A, consistent with CysNO inducing widespread S-nitrosylation, this treatment also caused disulfide oxidation of the redox-modulated kinases PKGIα and PKA RI. B, CysNO also efficiently induced disulfide formation in recombinant N-terminal PKGIα, demonstrating that this oxidation state can result as a direct reaction of the oxidizing NO donor and the kinase. This supports the possibility that CysNO may directly S-nitrosylate the kinases in vivo prior to self-reduction and consequential disulfide bond formation. C, by themselves, SNAP and cysteine failed to induce PKGIα or PKA RI interprotein disulfide bond formation. However, together, they efficiently induced disulfides into both kinases, consistent with SNAP S-nitrosylating cysteine extracellularly before transport into the cell, where it modifies proteins.